(厦门大学生命科学学院,细胞应激生物学国家重点实验室,福建 厦门 361102)
(State Key Laboratory of Cellular Stress Biology,School of Life Sciences,Xiamen University,Xiamen 361102,China)
stress granule; RNA binding protein; membraneless organelle; phase separation; neurodegenerative disease
DOI: 10.6043/j.issn.0438-0479.202110037
备注
应激颗粒是一类无膜细胞器,主要由RNA与RNA结合蛋白(RBP)组成.在受到外界压力刺激后,RBP结合相应的信使RNA,主要通过液-液相分离过程促进应激颗粒形成.应激颗粒的动态变化过程与神经退行性疾病密切相关.本文综述应激颗粒的组装、去组装及其参与神经退行性疾病的调控过程和机制,为这些疾病的有效治疗提供新的思路.
Background: RNA-binding proteins (RBPs) control mRNA metabolism during stress, in part through the formation of membraneless organelles. Stress granules (SGs) are assemblies of mRNA and proteins that form when mRNAs translation is stalled due to stress. These SGs form via a process known as the liquid-liquid phase separation (LLPS). LLPS has emerged as a fundamental mechanism to explain the formation of membraneless organelles. Recent advances suggest that the response of RNA metabolism to stress plays an important role in the pathophysiology of neurodegenerative diseases, in particular the amyotrophic lateral sclerosis (ALS), frontotemporal dementias (FTD) and Alzheimer disease (AD). Multiple biochemical pathways regulate SG biology. Despite the detailed knowledge of the composition and dynamics of SGs gained in the past, the function of SGs remains poorly understood.
Progress: The assembly of SGs is a multi-step process. Untranslated mRNA and SGs nucleating proteins are assembled by LLPS to form a dynamic macromolecular assembly. The structure consists of a core and a shell. Intrinsically disordered regions (IDRs) in the mRNA binding protein drive the formation of SGs through heteromorphic or isotypic interaction, and the multivalent interaction motifs and short linear motifs in the IDRs structure are usually subject to post-translational modifications. Dynamics are one of the most important characteristics of SGs. Most SGs behave like liquid, and their components are in dynamic balance with cytoplasm. The disassembly of SGs is accomplished through two possible pathways: autophagy-independent decomposition or autophagy-dependent degradation. In different types of neurodegenerative diseases such as ALS, FTD, AD and Parkinson's disease (PD), the underlying mechanisms of SG dynamics may be different. Exploring the role of SGs dynamics in neurodegenerative diseases is of great significance for the treatment of these diseases. In ALS/FTD patients, many RBP genes encoding SG constituents (e.g., TARDBP, FUS and ATXN2) are mutated, and all these mutations affect the dynamics of SGs. In addition, dysfunction of RBP affects the dynamic changes of SGs, which is an important contributing factor of ALS/FTD pathology. In recent years, more and more studies have begun to focus on the dynamic change of SGs involved in the deposition of Aβ-amyloid and the regulation of tau protein hyperphosphorylation in the AD patients. In PD patients, Dj-1 mutation leads to familial PD, and abnormal function of Dj-1 protein is closely related to altered dynamics of SGs. The dynamic changes of SGs are also involved in the regulation of other neurodegenerative diseases such as Creutzfeldt-Jakob disease (CJD) and Huntington’s disease (HD).
Perspective: SGs are membraneless organelles composed of mRNA and RBP. The role of SGs in cell has not been fully recognized so far. The focus of future research work is to decipher the proteome of SGs in different diseases and comprehensively understand the functions of SG proteins, so as to discover new drug targets. SGs are dynamic components, and their assembly and disassembly are regulated by various post-translational modifications. In this process, SGs proteins must undergo complex changes, which remain unclear at present. Preventing the generation of the pathological SGs and/or promoting the breakdown of these SGs has positive implications for the treatment of neurodegenerative diseases. In neurodegenerative diseases, TDP-43 protein or hyperphosphorylated Tau recruited into SGs will affect neuronal function and lead to neurodegenerative changes. Pharmacological interventions for protein misfolding and over-aggregation of SGs can alleviate some of the pathological phenotypes associated with neurodegeneration. These pathways may offer new drug targets and are important directions for future research on neurodegenerative diseases. Knowledge about the properties of SGs in various diseases and the molecular pathways involved in the biology of SGs is rapidly accumulating. Such information will contribute to the clinical application of SGs research and provide new directions and therapeutic targets for the treatment of various diseases.
引言
应激颗粒是细胞中的一类无膜细胞器,受外界刺激时细胞内整体翻译受到抑制,应激颗粒发生组装; 当外界刺激消除后,应激颗粒发生去组装[1].应激颗粒动态变化的异常会改变其生化或生物物理性质,可能导致多种慢性病变,特别是神经退行性疾病[1].本文以应激颗粒的动态变化为基点,聚焦其对神经退行性疾病发生发展的影响,以此阐述应激颗粒动态变化在神经退行性疾病中的致病机制.
1 应激颗粒的组装与去组装
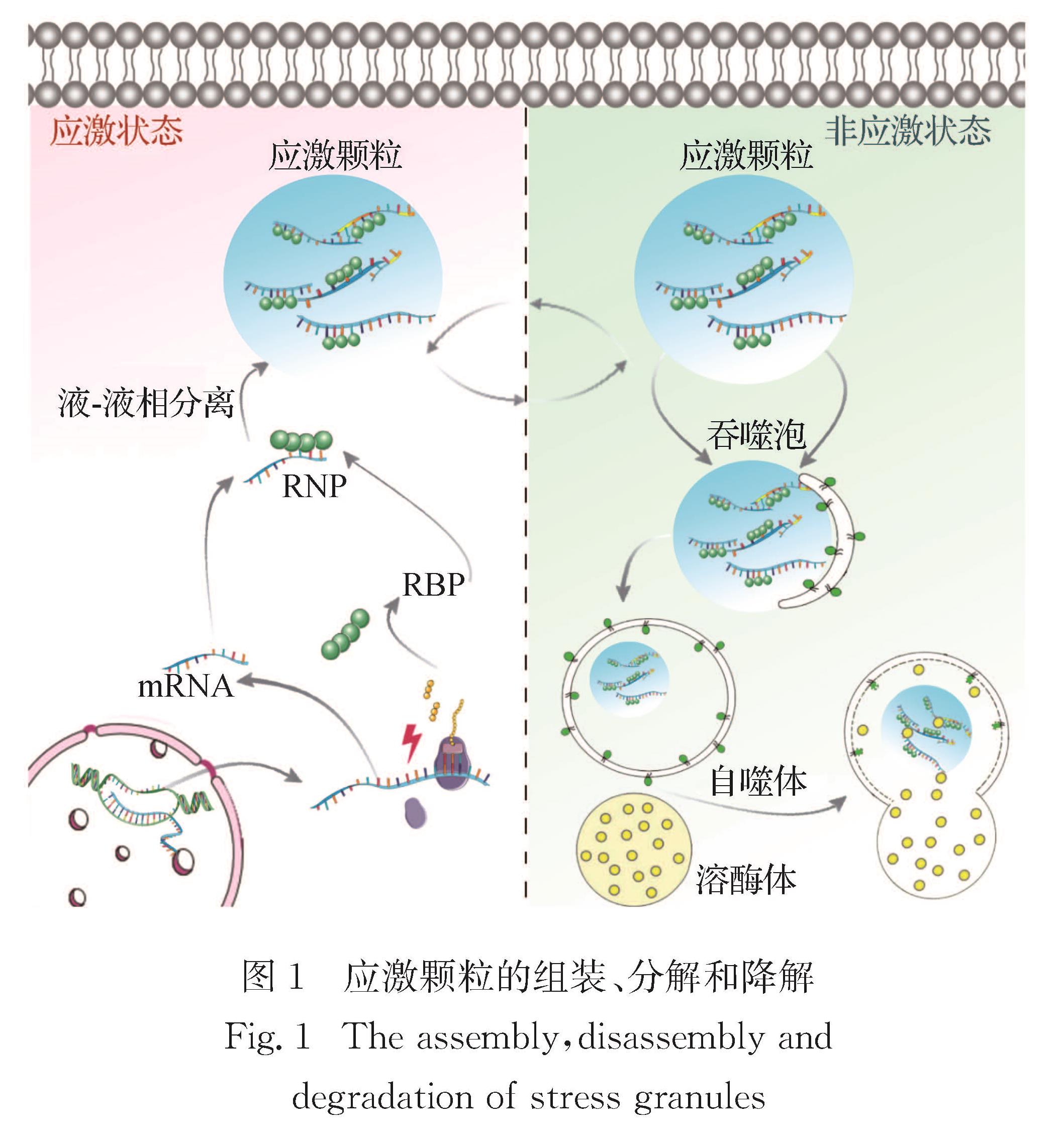
1.1 应激颗粒的组装应激颗粒是细胞质中的无膜结构,在各种环境压力(如热激、病毒感染等)下诱导产生[2],由翻译阻断的信使RNA(mRNA)与多种RNA结合蛋白(RBP)等组成[3].应激颗粒的组装是一个多步骤的过程:1)停滞的翻译起始复合物与多核糖体的分离[4]; 2)环境压力导致真核翻译起始因子(eIF2)α亚基的磷酸化,通过消耗eIF2·三磷酸鸟苷(GTP)复合物-Met-tRNAiMet三元复合物来抑制翻译起始,该复合物将启动子 Met-tRNAiMet加载到 AUG 起始密码子上[5],进而导致翻译停滞的48S预起始复合物(PIC)组装,并使得mRNA脱离下来[6]; 3)此外,应激颗粒的形成还需要应激颗粒成核蛋白[7](包括G3BP1、TIA1、TIAR、CSDE1和UBAP2L等[8])的参与.未翻译的mRNA与应激颗粒成核蛋白通过液-液相分离组装形成稳定的“核心”[9],进而招募更多的应激颗粒成核剂,在其周围形成更动态的外围“壳”状结构[10].这些“壳”包裹“核心”的核糖核蛋白生物大分子组装即是应激颗粒.
生物相分离主要由多价蛋白质相互作用域或内在无序区域(IDR)之间的相互作用所驱动[11-12].值得注意的是,mRNA 结合蛋白中的IDR通过异型或同型相互作用,驱动应激颗粒的形成[13-14],且IDR结构中的多价相互作用基序和短线性基序通常经过翻译后修饰[15-16].应激颗粒蛋白的翻译后修饰包含磷酸化、乙酰化、甲基化等[17-18],这些修饰会影响它们与应激颗粒其他组分的相互作用,进而调节整个颗粒的组装.研究表明:G3BP1的去磷酸化促进同源二聚化,从而促进应激颗粒的组装[19]; 锌指蛋白三四脯氨酸(TTP)或肉瘤融合蛋白(FUS)的磷酸化抑制了它们向应激颗粒的募集[20]; FUS低复杂性结构域的磷酸化可以阻碍应激颗粒的组装[21]; 脆性X综合征相关蛋白(FMRP)的甲基化能够促进FMRP与mRNA结合,形成应激颗粒[22]; Dead-box RNA解旋酶DDX3X的IDR去乙酰化,可增强其相分离的倾向[23].
动态性是应激颗粒最重要的特征之一.大多数应激颗粒表现出类似液体的行为,其成分与细胞质处于动态平衡[10].荧光漂白恢复(FRAP)实验表明:应激颗粒蛋白穿梭进出应激颗粒的过程中,单个蛋白的交换速率在几秒到几分钟范围内; TIA1、G3BP1和TTP的荧光信号在漂白后迅速恢复[3,24],而人类抗原R与多聚腺苷酸结合蛋白的FRAP半衰期相对较长[25-27].同时,应激颗粒形成的物理基础是液-液相分离.体外条件下,重组43 ku Tar DNA结合蛋白(TDP-43)、FUS和其他核不均一核糖核蛋白(hnRNP)能够通过IDR结构域之间相互作用发生相分离,自发地从水溶液中分离并形成富含蛋白质的液滴结构,这些液滴能够相互融合形成更大的液滴[28-29].在体内某些病理条件下,相应应激颗粒会产生相变,在病毒感染期间组装并起到隔离宿主和病毒的作用[30].新型冠状病毒的核衣壳蛋白在受感染细胞内高表达,提高了病毒 RNA 转录的效率[31],它能够与 G3BP1相互作用并破坏应激颗粒的组装,提高病毒的复制效率[30].在肌萎缩侧索硬化(ALS)中,约20%的家族性ALS与胞质蛋白超氧化物歧化酶1(SOD1)的错误折叠和聚集有关[32].SOD1的错误折叠,使其与人类细胞中的应激颗粒特异性地聚集,改变应激颗粒组成,触发应激颗粒中的液固相变,进而导致应激颗粒转变为病理包涵体[33].这些证据均表明应激颗粒具有动态性特征.
抑制翻译的药理学实验是研究应激颗粒组装过程的重要手段.当多核糖体因压力或药物干预而被分解时(如抗生素嘌呤霉素处理会导致蛋白质合成过早终止),细胞PIC 中未翻译的信使核糖核蛋白(mRNP)库增加,这有利于应激颗粒组装; 相反,翻译延伸的抑制不利于应激颗粒组装(如用放线菌酮处理,可干扰蛋白质合成的易位步骤,阻止翻译延伸,并使核糖体停留在mRNA 上)[34].应激颗粒的形成与细胞中的翻译状态紧密相关,应激颗粒与翻译控制之间的动态联系将应激颗粒与许多其他RNA颗粒区分开来.
1.2 应激颗粒的分解应激颗粒是高度动态的结构,在形成后的一段时间内会发生分解[35].在培养的人类细胞中发现,应激颗粒的去组装是通过两种可能的途径完成的,即不依赖自噬的分解和依赖自噬的降解[36].短寿命的应激颗粒去组装过程伴随着翻译的恢复,使细胞能够回收应激颗粒的组分,如蛋白质、mRNA等,从而避免了这些组分的从头合成[37].
应激颗粒的分解可以通过分子伴侣介导[25].热休克蛋白(HSP)是一大类分子伴侣,属于进化上保守的“应激反应”蛋白,可以促进细胞在各种不利条件下存活[38].HSP70家族与应激颗粒的分解有关[39],HSP70过表达能够阻止TIA1的聚集并促进应激细胞中应激颗粒的分解[40].同时,HSP70在识别易聚集(错误折叠)蛋白方面发挥着关键作用,一旦HSP70与目标蛋白结合后,它能够与HSPB8-Bcl2相关永生基因3(BAG3)形成复合物,及时分解应激颗粒[37],HSPB8-BAG3-HSP70复合物在应激颗粒分解中的功能为分子伴侣、mRNA代谢和细胞稳态之间的关键联系提供了重要证据[41-42].
最新研究表明,热休克诱导的泛素化是含缬酪肽蛋白(VCP/p97)介导的应激颗粒分解的先决条件[43].G3BP1的多泛素化使其与泛素依赖性分离酶VCP/p97的内质网相关接头Fas相关因子(FAF2)结合; 随后通过VCP从应激颗粒中提取G3BP1,导致它们的分解[36].此外,VCP在应激颗粒去组装中的功能还受到自噬诱导激酶ULK1/2的调节.在热激或氧化刺激下,ULK1/2特异性地磷酸化VCP,以一种不依赖于自噬的机制促进应激颗粒的去组装[44].
1.3 应激颗粒的降解应激颗粒的降解受到蛋白质质量控制(PQC)机制的调控.PQC机制由分子伴侣机制、泛素-蛋白酶体系统和自噬系统组成,它们能够识别错误折叠的蛋白质并促进其重新折叠或降解[33].自噬是细胞质或受损细胞器在细胞内降解的主要机制,涉及双膜结构的形成,该结构称为自噬体,它吞噬部分细胞质或细胞器并将其输送到溶酶体中进行降解[45].自噬可作为重要的PQC系统,用于消除与各种神经退行性疾病相关的病理产物,其中就包括应激颗粒,该过程被称为“粒噬”[46].在没有任何压力的情况下,观察到在敲除核心自噬基因Atg7的成纤维细胞中出现应激颗粒,而相应的野生型成纤维细胞则不含有应激颗粒,表明哺乳动物细胞中至少有一部分应激颗粒被自噬清除[46].
总的来说,多项研究证据表明分子伴侣、自噬和蛋白酶体以及它们的相互作用参与应激颗粒的清除过程(图1).
2 应激颗粒参与神经退行性疾病的调控
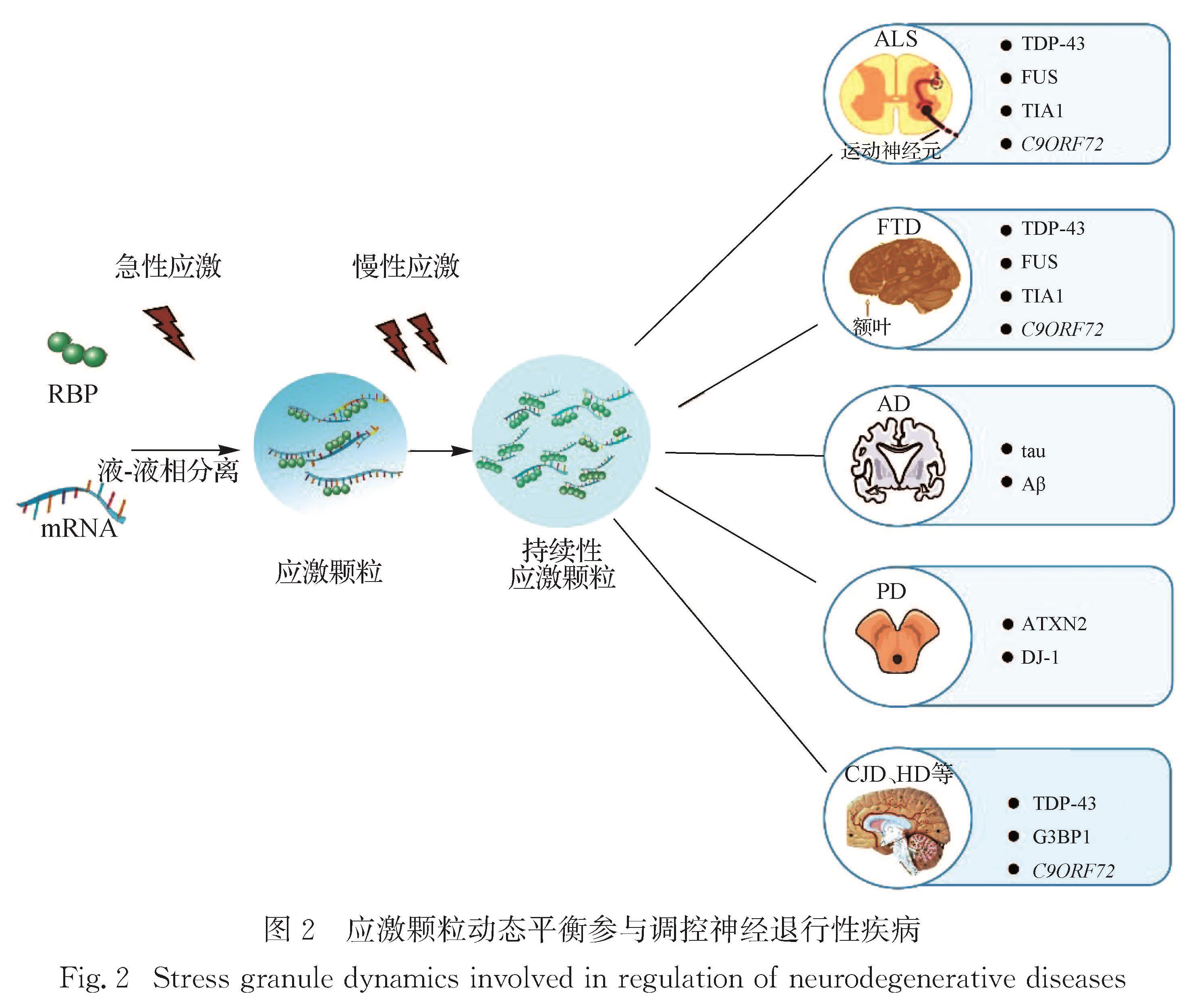
近年来,应激颗粒的动态变化在神经退行性疾病的研究中备受关注[29,47],然而其机制尚不明确.在不同类型的神经退行性疾病如ALS、额叶痴呆(FTD)、阿尔茨海默病(AD)、帕金森病(PD)中,应激颗粒的动态变化机制可能存在差异.探明应激颗粒动态变化的作用对治疗这些疾病具有重大意义.
2.1 应激颗粒与ALS/FTDALS是一种破坏性的运动神经疾病,会导致四肢瘫痪,甚至致命的横膈膜瘫痪[48-49].FTD临床表现为进行性认知、行为和语言障碍[50].这两种神经退行性疾病的临床表型及致病机制有颇多相似之处[51-52],共同病理特征主要为:RBP异常聚集、RNA代谢受损、RNP颗粒异常形成和自噬相关蛋白累积等[53-55].
在ALS/FTD患者中,许多编码应激颗粒相关RBP的基因(如TARDBP、FUS和ATXN2)存在突变[56-57].研究表明,家族性及部分散发性ALS与TARDBP基因(编码TDP-43蛋白)突变相关[58].TDP-43R361S功能缺失突变会降低G3BP1的表达,同时增加TIA1的表达,导致应激颗粒形成缓慢,并更快地完成去组装[59].FUS基因的突变导致细胞核中FUS蛋白在细胞质中错误定位,并促进应激颗粒的形成[60].在携带FUS基因突变的患者中,也发现运动神经元退化,提示FUS基因的突变可能是造成ALS/FTD运动神经元损伤的重要因素[56].另外,ALS/FTD患者中也发现失调症蛋白(ATXN2/Ataxin-2)的突变,Ataxin-2多聚谷氨酰胺重复表达可能与TDP-43异常聚集相关,通过调节TDP-43聚集物的相分离,从而影响应激颗粒形成[61-62].在ALS/FTD患者中也发现了TIA1的突变(P362L),该突变发生在IDR,可促进TIA1的相分离,并影响应激颗粒动态平衡; 进一步在活细胞中发现该突变延迟了应激颗粒分解,可促进TDP-43聚集物发生相分离,加快应激颗粒组装[63].
RBP功能异常影响应激颗粒的动态变化,是导致ALS/FTD病变的重要因素[64].近些年,科学家们在ALS/FTD患者脊椎的病理学切片中发现TDP-43异常聚集,这已经成为鉴定ALS/FTD的标准之一[65].TDP-43的异常聚集物会不断被招募到应激颗粒中,后者进一步促进TDP-43在细胞质中累积,提示该过程可能也是ALS/FTD持久致病的原因之一[66]; TDP-43功能的缺失会破坏正常的应激颗粒动态平衡,也可能导致ALS患者神经元损伤[59].ALS/FTD患者的另一重要病理特征是FUS在细胞质中的异常聚集,这种聚集物会被招募到应激颗粒中,促进应激颗粒组装[67].此外,核质运输的缺陷会导致TDP-43与FUS在细胞质中的错误定位,促进应激颗粒组装[68-69].值得一提的是,平面结构的芳香族化合物(如茴香霉素)可以阻止ALS相关RBP(如TDP-43和FUS)在应激颗粒中的富集,这类化合物也可以减少ALS患者运动神经元中TDP-43的积累[66].
除RBP功能异常外,其他因素也可参与应激颗粒动态变化的调控,进而影响ALS/FTD.第9号染色体开放性阅读框72(C9ORF72)基因中的重复碱基序列GGGGCC(G4C2)异常翻译的二肽重复蛋白(DRP)是ALS/FTD的重要致病因素之一[67,70].DRP也可以调控应激颗粒的动态变化,如含精氨酸的DRP(poly Gly-Arg)与含IDR的RBP(如TDP-43和FUS)相互作用,改变其相分离,从而影响应激颗粒的动力学,这也是造成ALS/FTD发生的原因之一[70].血管生成素(ANG)是哺乳动物细胞中分泌的一种核糖核酸内切酶,它能切割转运RNA以启动应激反应[71].近期研究显示,ANG的突变与ALS密切相关,ANG可以促进亚砷酸盐和pateamine A诱导的应激颗粒组装[71].
应激颗粒的降解需要完整的自噬,而神经退行性疾病患者的病变细胞往往存在自噬活性失调,有可能导致应激颗粒降解受损[72].越来越多的证据表明,ALS/FTD相关自噬受体蛋白(如SQSTM1/p62、Optineurin和Ubiquilin-2)突变可能影响应激颗粒降解过程[72-73].当SQSTM1/p62缺失时,自噬诱导应激颗粒的降解被阻断,进而导致运动神经元受损[74].在ALS/FTD患者中也存在大量的AAA三磷酸腺苷酶(VCP)突变[75].VCP通过水解三磷酸腺苷来加速生物大分子复合物解聚,同时也能调节自噬[76].目前研究较为清楚的VCP突变包括R155H和A232E等,病变细胞均表现出受损的自噬活性[77].此外,在表达这些VCP突变的细胞中积累大量的应激颗粒,暗示自噬在降解应激颗粒过程中扮演重要角色[44,46].
外在环境因素可能影响应激颗粒的动态变化.外伤性脑损伤(TBI)是诱发ALS/FTD的重要外部因素[78-79].在果蝇(Drosophila melanogaster)ALS/FTD模型中的研究发现,诱发TBI可诱导果蝇大脑应激颗粒的组装,这些应激颗粒中存在泛素、p62和TDP-43等成分,同时这些果蝇表现出运动型损伤等ALS/FTD类似表型,提示创伤可以在体内诱导应激颗粒的形成,并可能增强神经退行表型[80].
以上证据表明,多种因素都可以导致应激颗粒动态异常,从而影响ALS/FTD的进程.
2.2 应激颗粒与ADAD是一种最常见的与记忆衰退相关的神经退行性疾病,然而目前尚无有效治疗方法[81].一般认为,Aβ-样淀粉的沉积和tau蛋白的过度磷酸化是AD的主要致病因素[82].有研究发现在AD模型小鼠的海马区存在应激颗粒聚集,且主要定位于神经元中[83].近年来,越来越多的研究聚焦于应激颗粒动态变化参与的Aβ-样淀粉沉积和tau蛋白过度磷酸化的调控[81].
应激颗粒的动态变化与tau蛋白的生理和病理密切相关[84].在生理条件下,过表达tau蛋白可促进神经元细胞系和原代神经元培养中应激颗粒的形成; 而在病理情况下,编码tau蛋白的MAPT基因突变会导致更大、更稳定的应激颗粒形成,tau蛋白的过度磷酸化可能在这一途径中发挥重要作用[84].这提示应激颗粒的代谢延长可能是影响AD病变的重要因素.
随着AD病变的发展,应激颗粒长期、稳定存留在细胞质中不被降解[85].抑制应激颗粒的组装可能缓解AD的病变[86]:TIA1和tau存在相互作用,tau蛋白可以调控TIA1的分布,同时TIA1过表达也可以促进tau蛋白的磷酸化及错误折叠,后者可进一步加速应激颗粒的形成并诱导神经退化; 敲除TIA1基因可抑制tau蛋白的错误折叠及其毒性.在转基因P301S tau小鼠中,TIA1基因缺失可抑制应激颗粒形成,同时也可减少tau蛋白的聚集,缓解转基因P301S tau小鼠的神经退行性病变.
在Aβ-样淀粉沉积诱导的AD患者中,慢性压力会诱导应激颗粒相关的RBP(如酵母丙酮酸激酶的主要亚型Cdc19)演变成非功能性淀粉样蛋白,并发生病理性聚集,被招募到应激颗粒中[87].另外,在AD模型小鼠中发现,蛋白激酶GCN2的缺失可以防止AD模型小鼠神经元突触可塑性损伤和空间记忆缺陷,这一效果可能是通过抑制应激颗粒形成达到的[88].
目前在AD中,对应激颗粒动态调控的研究主要聚焦于tau蛋白磷酸化的调控,抑制应激颗粒的形成被认为是一种治疗AD的新方法[81,83].然而针对Aβ-样淀粉沉积与应激颗粒动态关系及机制的研究欠缺,未来对此的研究或许可为AD的治疗提供更多思路.
2.3 应激颗粒与PDPD目前主要认为是由黑质区域神经元异常多巴胺的释放以及 α-突触核蛋白在脑内的异常表达引起的[89].目前PD与应激颗粒动态变化的机制尚不明确,下文主要阐述PD与应激颗粒动态变化的联系.
由PD相关蛋白7(PARK7)基因编码的DJ-1突变会导致家族性PD发生,而DJ-1蛋白功能异常[90-91]与应激颗粒动态变化密切相关[92-93]:DJ-1可与哺乳动物细胞中的应激颗粒组分(如hnRNP)发生相互作用,在渗透或氧化刺激诱导下会被招募到应激颗粒中.进而通过纯化哺乳动物细胞中的mRNA发现,在受到刺激时谷胱甘肽过氧化物酶4(GPX4)、真核翻译起始因子4B(eIF4B)及其结合蛋白eIF4EBP1等基因的mRNA 会定位于应激颗粒中.值得注意的是,DJ-1与PD小鼠模型中神经元的应激颗粒有关[94].因此推测应激颗粒动态变化可能在DJ-1介导的PD中发挥重要作用.ATXN2基因突变(CAG碱基重复序列数≥32)可能是PD的罕见致病因素[94-95].最近一项研究报道,应激颗粒元件STAU1可在ATXN2基因突变的PD动物模型中富集,并促进应激颗粒的形成[95].
另外,研究由农药(避蚊胺、fipronil和maneb)引发的PD模型发现[96-97],这3种农药均可诱导应激颗粒的组装[71].长期接触农药可能形成稳定的应激颗粒,并最终转化为不溶性纤维聚集物,从而破坏应激反应途径,引起细胞死亡[98-99].相比于ALS与AD,PD与应激颗粒的相关研究较少,且有报道称应激颗粒似乎与PD并没有直接联系[85]; 尽管有研究认为农药诱导的PD可能与应激颗粒存在直接联系[100],但其证据尚不充分.因此,以应激颗粒为基点开展对PD的研究仍有待加强.
2.4 应激颗粒与其他神经退行性疾病应激颗粒动态变化也参与调控其他神经退行性疾病,如克雅二氏病(CJD)和亨廷顿氏病(HD)等.
20世纪90年代的研究发现,在CJD患者的球囊神经元细胞质中存在α-B-晶体蛋白的沉积[101],而这些蛋白沉积可能与应激颗粒形成相关[102].最近的研究发现,在散发性CJD患者中存在C9ORF72基因突变,并导致TDP-43和DRP聚集,促进应激颗粒的组装[103].
HD患者脑脊液中,大量G3BP1会被招募到应激颗粒中[104].在R6/2 转基因小鼠的皮层和海马区以及HD患者大脑的上额叶皮层中,G3BP1阳性应激颗粒的数量显著增加[104].研究者还观察到在HD皮质神经元中,TDP-43被错误定位到 G3BP1阳性应激颗粒中,促进应激颗粒的组装[104].
3 总结与展望
生理条件下应激反应是短暂的,但与衰老及相关神经退行性病变的慢性应激会导致应激颗粒缓慢、持续性生成,这些应激颗粒可能充当疾病相关蛋白聚集的核心[85].尽管应激颗粒的
图2 应激颗粒动态平衡参与调控神经退行性疾病
Fig.2 Stress granule dynamics involved in regulation of neurodegenerative diseases动态变化在不同神经退行性疾病中的机制有所差异[81,102,104-107](图2),但这些病理机制的共同点往往表现在应激颗粒的组装与去组装过程中[108].因此操纵应激颗粒动态过程的方法可能成为研究甚至治疗神经退行性疾病的手段.
应激颗粒是由mRNA和RBP组成的无膜细胞器.目前应激颗粒在细胞功能中的作用尚未得到充分认识.未来研究工作的重点是破译应激颗粒的蛋白质组,全面了解应激颗粒蛋白,进而定义不同疾病中应激颗粒的蛋白质组,以发现新的药物靶点.
应激颗粒是一种动态组件,其组装和去组装受许多翻译后修饰的调控,在此过程中应激颗粒蛋白发生了复杂的变化,但相关研究目前尚不全面.进一步了解应激颗粒动力学如何影响其功能,以及这些蛋白中的结构域如何在应激颗粒的动力学中发挥作用至关重要.阻止这些应激颗粒的产生或促进应激颗粒的分解,对于神经退行性疾病的治疗具有积极意义.错误折叠的蛋白与有缺陷的核糖体产物的聚集可以参与应激颗粒的形成,如TDP-43或过度磷酸化的tau蛋白募集到应激颗粒中会影响神经元功能,并导致神经退行性病变的发生[63],因此对于蛋白错误折叠和应激颗粒过度聚集的药理学干预能够缓解部分与神经变性相关的病理表型.这些途径可能揭示了新的药物靶点,是未来研究神经退行性病变的重要方向.
过去的研究发现,应激颗粒不仅是mRNA储存的位点,也可作为信号枢纽多方面影响细胞代谢,对癌症的发生发展至关重要[109].应激颗粒可能调节哺乳动物雷帕霉素靶点激酶的活性和定位,这是细胞代谢和生长的关键调节剂,与癌症的发生密切相关[110].虽然应激颗粒影响癌症的确切机制在很大程度上是未知的,但是可以推测应激颗粒的研究是未来癌症治疗的研究靶点之一.与此同时,应激颗粒是各种癌症潜在的诊断和预后生物标志物,以计算机结合荧光定量的方式分析肿瘤细胞中应激颗粒的组装和分解[111],该方法的可行性已在临床肿瘤样本中得到证实[112].因此,应激颗粒在临床应用中的开发也是未来研究的一个重要方向.
病毒感染可以诱导或抑制应激颗粒组装[113].如脊髓灰质炎病毒会阻止应激颗粒形成以应对氧化环境[114],但目前具体的机制还不清楚.通过操纵应激颗粒的形成发挥抗病毒的作用,可能是未来抗病毒治疗的策略之一.
目前,关于各种疾病中应激颗粒的特性以及应激颗粒生物学中所涉及分子途径的知识正在迅速积累.这些信息将有助于将应激颗粒的研究应用于临床,为各种疾病的治疗提供新的方向与靶点.
- [1] ASADI M R,SADAT MOSLEHIAN M,SABAIE H,et al.Stress granules and neurodegenerative disorders:a scoping review[J].Front Aging Neurosci,2021,13:650740.
- [2] BUCHAN J R,PARKER R.Eukaryotic stress granules:the ins and outs of translation[J].Molecular Cell,2009,36(6):932-941.
- [3] KEDERSHA N,STOECKLIN G,AYODELE M,et al.Stress granules and processing bodies are dynamically linked sites of mRNP remodeling[J].J Cell Biol,2005,169(6):871-884.
- [4] BOUNEDJAH O,DESFORGES B,WU T D,et al.Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules[J].Nucleic Acids Res,2014,42(13):8678-8691.
- [5] MERRICK W C,PAVITT G D.Protein synthesis initiation in eukaryotic cells[J].Cold Spring Harb Perspect Biol,2018,10(12):a033092.
- [6] NANCY K,SAMANTHA C,NATALIE G,et al.Evidence that ternary complex(eIF2·GTP-tRNAMet/sub>i)-deficient preinitiation complexes are core constituents of mammalian stress granules[J].Mol Biol Cell,2002,13(1):195-210.
- [7] PANAS M D,IVANOV P,ANDERSON P.Mechanistic insights into mammalian stress granule dynamics[J].J Cell Biol,2016,215(3):313-323.
- [8] ANDERSON P,KEDERSHA N.Stress granules:the Tao of RNA triage[J].Trends Biochem Sci,2008,33(3):141-150.
- [9] LIN Y,PROTTER D S W,ROSEN M K,et al.Formation and maturation of phase-separated liquid droplets by RNA-binding proteins[J].Mol Cell,2015,60(2):208-219.
- [10] WHEELER J R,MATHENY T,JAIN S,et al.Distinct stages in stress granule assembly and disassembly[J].eLife,2016,5:e18413.
- [11] LI P L,BANJADE S,CHENG H C,et al.Phase transitions in the assembly of multivalent signalling proteins[J].Nature,2012,483(7389):336-340.
- [12] UVERSKY V N.The multifaceted roles of intrinsic disorder in protein complexes[J].FEBS Lett,2015,589(19):2498-2506.
- [13] ZHANG H,ELBAUM-GARFINKLE S,LANGDON E M,et al.RNA controls polyQ protein phase transitions[J].Mol Cell,2015,60(2):220-230.
- [14] WEBER S C,BRANGWYNNE C P.Getting RNA and protein in phase[J].Cell,2012,149(6):1188-1191.
- [15] BAH A,FORMAN-KAY J D.Modulation of intrinsically disordered protein function by post-translational modifications[J].J Biol Chem,2016,291(13):6696-6705.
- [16] XIE H B,VUCETIC S,IAKOUCHEVA L M,et al.Functional anthology of intrinsic disorder.3.Ligands,post-translational modifications,and diseases associated with intrinsically disordered proteins[J].J Proteome Res,2007,6(5):1917-1932.
- [17] XIE W,DENMAN R B.Protein methylation and stress granules:posttranslational remodeler or innocent bystander?[J].Mol Biol Int,2011,2011:137459.
- [18] OHN T,KEDERSHA N,HICKMAN T,et al.A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly[J].Nat Cell Biol,2008,10(10):1224-1231.
- [19] TOURRIÈRE H,CHEBLI K,ZEKRI L,et al.The RasGAP-associated endoribonuclease G3BP assembles stress granules[J].J Cell Biol,2003,160(6):823-831.
- [20] REINEKE L C,TSAI W C,JAIN A,et al.Casein kinase 2 is linked to stress granule dynamics through phosphory-lation of the stress granule nucleating protein G3BP1[J].Mol Cell Biol,2017,37(4):e00596-16.
- [21] HAN T W,KATO M,XIE S H,et al.Cell-free formation of RNA granules:bound RNAs identify features and components of cellular assemblies[J].Cell,2012,149(4):768-779.
- [22] DOLZHANSKAYA N,MERZ G,ALETTA J M,et al.Methylation regulates the intracellular protein-protein and protein-RNA interactions of FMRP[J].J Cell Sci,2006,119(Pt9):1933-1946.
- [23] SAITO M,HESS D,EGLINGER J,et al.Acetylation of intrinsically disordered regions regulates phase separation[J].Nat Chem Biol,2019,15(1):51-61.
- [24] BUCHAN J R,PARKER R.Eukaryotic stress granules:the ins and outs of translation[J].Mol Cell,2009,36(6):932-941.
- [25] BUCHAN J R.mRNP granules.Assembly,function,and connections with disease[J].RNA Biol,2014,11(8):1019-1030.
- [26] GUIL S,LONG J C,CÁCERES J F.hnRNP A1 relocalization to the stress granules reflects a role in the stress response[J].Mol Cell Biol,2006,26(15):5744-5758.
- [27] MOLLET S,COUGOT N,WILCZYNSKA A,et al.Translationally repressed mRNA transiently cycles through stress granules during stress[J].Mol Biol Cell,2008,19(10):4469-4479.
- [28] CONICELLA A E,ZERZE G H,MITTAL J,et al.ALS mutations disrupt phase separation mediated by α-helical structure in the TDP-43 low-complexity C-terminal domain[J].Structure,2016,24(9):1537-1549.
- [29] MOLLIEX A,TEMIROV J,LEE J H,et al.Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization[J].Cell,2015,163(1):123-133.
- [30] LUO L L,LI Z A,ZHAO T J,et al.SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production[J].Sci Bull,2021,66(12):1194-1204.
- [31] SAVASTANO A,OPAKUA A,RANKOVIC M,et al.Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates[J].Nat Commun,2020,11(1):6041.
- [32] ROBBERECHT W,PHILIPS T.The changing scene of amyotrophic lateral sclerosis[J].Nat Rev Neurosci,2013,14(4):248-264.
- [33] MATEJU D,FRANZMANN T M,PATEL A,et al.An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function[J].EMBO J,2017,36(12):1669-1687.
- [34] KEDERSHA N,CHO M R,LI W,et al.Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules[J].J Cell Biol,2000,151(6):1257-1268.
- [35] KEDERSHA N L,GUPTA M,LI W,et al.RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules[J].J Cell Biol,1999,147(7):1431-1442.
- [36] GWON Y,MAXWELL B A,KOLAITIS R M,et al.Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner[J].Science,2021,372(6549):eabf6548.
- [37] ALBERTI S,MATEJU D,MEDIANI L,et al.Granu-lostasis:protein quality control of RNP granules[J].Front Mol Neurosci,2017,10:84.
- [38] VERMA A,SUMI S,SEERVI M.Heat shock proteins-driven stress granule dynamics:yet another avenue for cell survival[J].Apoptosis,2021,26(7/8):371-384.
- [39] MAZROUI R,DI MARCO S,KAUFMAN R J,et al.Inhibition of the ubiquitin-proteasome system induces stress granule formation[J].Mol Biol Cell,2007,18(7):2603-2618.
- [40] GILKS N,KEDERSHA N,AYODELE M,et al.Stress granule assembly is mediated by prion-like aggregation of TIA-1[J].Mol Biol Cell,2004,15(12):5383-5398.
- [41] WALTERS R W,PARKER R.Coupling of ribostasis and proteostasis:Hsp70 proteins in mRNA metabolism[J].Trends Biochem Sci,2015,40(10):552-559.
- [42] GANASSI M,MATEJU D,BIGI I,et al.A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity and dynamism[J].Mol Cell,2016,63(5):796-810.
- [43] MAXWELL B A,GWON Y,MISHRA A,et al.Ubiqui-tination is essential for recovery of cellular activities after heat shock[J].Science,2021,372(6549):eabc3593.
- [44] WANG B,MAXWELL B A,JOO J H,et al.ULK1 and ULK2 regulate stress granule disassembly through phosphorylation and activation of VCP/p97[J].Mol Cell,2019,74(4):742-757.
- [45] ZHANG Y X,YAN L B,ZHOU Z,et al.SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C.elegans[J].Cell,2009,136(2):308-321.
- [46] BUCHAN J R,KOLAITIS R M,TAYLOR J P,et al.Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function[J].Cell,2013,153(7):1461-1474.
- [47] PATEL A,LEE H O,JAWERTH L,et al.A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation[J].Cell,2015,162(5):1066-1077.
- [48] BROOKS B R,MILLER R G,SWASH M,et al.El escorial revisited:revised criteria for the diagnosis of amyotrophic lateral sclerosis[J].Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders,2000,1(5):293-299.
- [49] TANDAN R,BRADLEY W G.Amyotrophic lateral sclerosis:part 1.Clinical features,pathology,and ethical issues in management[J].Ann Neurol,1985,18(3):271-280.
- [50] MACKENZIE I R A,NEUMANN M.Molecular neuro-pathology of frontotemporal dementia:insights into disease mechanisms from postmortem studies[J].J Neu-rochem,2016,138(Sup.1):54-70.
- [51] CRUTS M,GIJSELINCK I,VAN LANGENHOVE T,et al.Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum[J].Trends in Neurosciences,2013,36(8):450-459.
- [52] MACKENZIE I R A.The neuropathology of FTD associated with ALS[J].Alzheimer Dis Assoc Disord,2007,21(4):S44-S49.
- [53] MANDRIOLI J,MEDIANI L,ALBERTI S,et al.ALS and FTD:where RNA metabolism meets protein quality control[J].Semin Cell Dev Biol,2020,99:183-192.
- [54] LI Y R,KING O D,SHORTER J,et al.Stress granules as crucibles of ALS pathogenesis[J].J Cell Biol,2013,201(3):361-372.
- [55] SIDIBÉ H,KHALFALLAH Y,XIAO S X,et al.TDP-43 stabilizes G3BP1 mRNA:relevance to amyotrophic lateral sclerosis/frontotemporal dementia[J].Brain,2021,144(11):3461-3476.
- [56] VANCE C,ROGELJ B,HORTOBÁGYI T,et al.Mutations in FUS,an RNA processing protein,cause familial amyotrophic lateral sclerosis type 6[J].Science,2009,323(5918):1208-1211.
- [57] SREEDHARAN J,BLAIR I P,TRIPATHI V B,et al.TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis[J].Science,2008,319(5870):1668-1672.
- [58] KABASHI E,VALDMANIS P N,DION P,et al.TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis[J].Nat Genet,2008,40(5):572-574.
- [59] MCDONALD K K,AULAS A,DESTROISMAISONS L,et al.TAR DNA-binding protein 43(TDP-43)regulates stress granule dynamics via differential regulation of G3BP and TIA-1[J].Hum Mol Genet,2011,20(7):1400-1410.
- [60] LIAO S K,KAWAKUBO Y,SMITH J W,et al.Extent of morphological differentiation of human melanoma cells reflected by HLA-DR antigen profiles[J].Prog Clin Biol Res,1985,172B:355-366.
- [61] BARADARAN-HERAVI Y,VAN BROECKHOVEN C,VAN DER ZEE J.Stress granule mediated protein aggregation and underlying gene defects in the FTD-ALS spectrum[J].Neurobiol Dis,2020,134:104639.
- [62] ELDEN A C,KIM H J,HART M P,et al.Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS[J].Nature,2010,466(7310):1069-1075.
- [63] MACKENZIE I R,NICHOLSON A M,SARKAR M,et al.TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics[J].Neuron,2017,95(4):808-816.
- [64] SAMARAWEERA R N,FELDMAN L,WIDRICH W C,et al.Stomal varices:percutaneous transhepatic embolization[J].Radiology,1989,170(3Pt1):779-782.
- [65] NEUMANN M,SAMPATHU D M,KWONG L K,et al.Ubiquitinated TDP-43 in frontotemporal lobar dege-neration and amyotrophic lateral sclerosis[J].Science,2006,314(5796):130-133.
- [66] FANG M Y,MARKMILLER S,VU A Q,et al.Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD[J].Neuron,2019,103(5):802-819.
- [67] HOFWEBER M,HUTTEN S,BOURGEOIS B,et al.Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation[J].Cell,2018,173(3):706-719.
- [68] DORMANN D,HAASS C.TDP-43 and FUS:a nuclear affair[J].Trends Neurosci,2011,34(7):339-348.
- [69] ITO D,SUZUKI N.Conjoint pathologic cascades mediated by ALS/FTLD-U linked RNA-binding proteins TDP-43 and FUS[J].Neurology,2011,77(17):1636-1643.
- [70] LEE K H,ZHANG P P,KIM H J,et al.C9orf72 dipeptide repeats impair the assembly,dynamics,and function of membrane-less organelles[J].Cell,2016,167(3):774-788.
- [71] EMARA M M,IVANOV P,HICKMAN T,et al.Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly[J].J Biol Chem,2010,285(14):10959-10968.
- [72] SEGUIN S J,MORELLI F F,VINET J,et al.Inhibition of autophagy,lysosome and VCP function impairs stress granule assembly[J].Cell Death Differ,2014,21(12):1838-1851.
- [73] MAJCHER V,GOODE A,JAMES V,et al.Autophagy receptor defects and ALS-FTLD[J].Mol Cell Neurosci,2015,66(PtA):43-52.
- [74] CHITIPROLU M,JAGOW C,TREMBLAY V,et al.A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy[J].Nat Commun,2018,9(1):2794.
- [75] TAYLOR J P.Multisystem proteinopathy:intersecting genetics in muscle,bone,and brain degeneration[J].Neurology,2015,85(8):658-660.
- [76] MEYER H,WEIHL C C.The VCP/p97 system at a glance:connecting cellular function to disease pathogenesis[J].J Cell Sci,2014,127(Pt18):3877-3883.
- [77] TRESSE E,SALOMONS F A,VESA J,et al.VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD[J].Autophagy,2010,6(2):217-227.
- [78] PUPILLO E,MESSINA P,LOGROSCINO G,et al.Trauma and amyotrophic lateral sclerosis:a case-control study from a population-based registry[J].Eur J Neurol,2012,19(12):1509-1517.
- [79] MORTIMER J A,VAN DUIJN C M,CHANDRA V,et al.Head trauma as a risk factor for Alzheimer's disease:a collaborative re-analysis of case-control studies[J].Int J Epidemiol,1991,20(Sup.2):S28-S35.
- [80] ANDERSON E N,GOCHENAUR L,SINGH A,et al.Traumatic injury induces stress granule formation and enhances motor dysfunctions in ALS/FTD models[J].Hum Mol Genet,2018,27(8):1366-1381.
- [81] SHEN Y,ZHANG T,ZHANG Y L,et al.Stress granules modulate SYK to cause tau-associated neurocognitive deterioration in 5XFAD mouse after anesthesia and surgery[J].Front Aging Neurosci,2021,13:718701.
- [82] SCHELTENS P,DE STROOPER B,KIVIPELTO M,et al.Alzheimer's disease[J].The Lancet,2021,397(10284):1577-1590.
- [83] FRYDRY 'KOV K,MAEK T,POSPÍEK M.Changing faces of stress:impact of heat and arsenite treatment on the composition of stress granules[J].Wiley Interdiscip Rev RNA,2020,11(6):e1596.
- [84] VANDERWEYDE T,APICCO D J,YOUMANS-KIDDER K,et al.Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity[J].Cell Rep,2016,15(7):1455-1466.
- [85] WOLOZIN B,IVANOV P.Stress granules and neuro-degeneration[J].Nat Rev Neurosci,2019,20(11):649-666.
- [86] APICCO D J,ASH P E A,MAZIUK B,et al.Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo[J].Nat Neurosci,2018,21(1):72-80.
- [87] GRIGNASCHI E,CEREGHETTI G,GRIGOLATO F,et al.A hydrophobic low-complexity region regulates aggregation of the yeast pyruvate kinase Cdc19 into amyloid-like aggregates in vitro[J].J Biol Chem,2018,293(29):11424-11432.
- [88] MA T,TRINH M A,WEXLER A J,et al.Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits[J].Nat Neurosci,2013,16(9):1299-1305.
- [89] JUCKER M,WALKER L C.Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases[J].Nat Neurosci,2018,21(10):1341-1349.
- [90] ARIGA H,TAKAHASHI-NIKI K,KATO I,et al.Neuroprotective function of DJ-1 in Parkinson's disease[J].Oxid Med Cell Longev,2013,2013:683920.
- [91] ZHANG H,DUAN C,YANG H.Defective autophagy in Parkinson's disease:lessons from genetics[J].Mol Neurobiol,2015,51(1):89-104.
- [92] BONIFATI V,BREEDVELD G J,SQUITIERI F,et al.Localization of autosomal recessive early-onset Parkin-sonism to chromosome 1p36(PARK7)in an independent dataset[J].Ann Neurol,2002,51(2):253-256.
- [93] BONIFATI V,RIZZU P,VAN BAREN M J,et al.Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism[J].Science,2003,299(5604):256-259.
- [94] REPICI M,HASSANJANI M,MADDISON D C,et al.The Parkinson's disease-linked protein DJ-1 associates with cytoplasmic mRNP granules during stress and neurodegeneration[J].Mol Neurobiol,2019,56(1):61-77.
- [95] PAUL S,DANSITHONG W,FIGUEROA K P,et al.Staufen1 in human neurodegeneration[J].Ann Neurol,2021,89(6):1114-1128.
- [96] TINAKOUA A,BOUABID S,FAGGIANI E,et al.The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat[J].Neuroscience,2015,311:118-129.
- [97] PARK J H,PARK Y S,KOH H C.Progressive loss of nigrostriatal dopaminergic neurons induced by inflammatory responses to fipronil[J].Toxicol Lett,2016,258:36-45.
- [98] ADVANI V M,IVANOV P.Stress granule subtypes:an emerging link to neurodegeneration[J].Cell Mol Life Sci,2020,77(23):4827-4845.
- [99] WANG F,LI J,FAN S J,et al.Targeting stress granules:a novel therapeutic strategy for human diseases[J].Pharmacol Res,2020,161:105143.
- [100] BHADAURIYA P,PARIHAR R,GANESH S.Pesticides DEET,fipronil and maneb induce stress granule assembly and translation arrest in neuronal cells[J].Biochem Biophys Rep,2021,28:101110.
- [101] KATO S,HIRANO A,UMAHARA T,et al.Ultra-structural and immunohistochemical studies on ballooned cortical neurons in Creutzfeldt-Jakob disease:expression of αB-crystallin,ubiquitin and stress-response protein 27[J].Acta Neuropathol,1992,84(4):443-448.
- [102] DOERWALD L,VAN GENESEN S T,ONNEKINK C,et al.The effect of αB-crystallin and Hsp27 on the availability of translation initiation factors in heat-shocked cells[J].Cell Mol Life Sci,2006,63(6):735-743.
- [103] KLOTZ S,KÖNIG T,ERDLER M,et al.Co-incidental C9orf72 expansion mutation-related frontotemporal lobar degeneration pathology and sporadic Creutzfeldt-Jakob disease[J].Eur J Neurol,2021,28(3):1009-1015.
- [104] SANCHEZ I I,NGUYEN T B,ENGLAND W E,et al.Huntington's disease mice and human brain tissue exhibit increased G3BP1 granules and TDP43 mislocalization[J].J Clin Invest,2021,131(12):e140723.
- [105] SUN Y L,CHAKRABARTTY A.Phase to phase with TDP-43[J].Biochemistry,2017,56(6):809-823.
- [106] VANDERWEYDE T,YU H,VARNUM M,et al.Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies[J].J Neurosci,2012,32(24):8270-8283.
- [107] OBA D,HAYASHI M,MINAMITANI M,et al.Autopsy study of cerebellar degeneration in siblings with ataxia-telangiectasia-like disorder[J].Acta Neuro-pathol,2010,119(4):513-520.
- [108] BOWLES K R,SILVA M C,WHITNEY K,et al.ELAVL4,splicing,and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids[J].Cell,2021,184(17):4547-4563.
- [109] ANDERSON P,KEDERSHA N,IVANOV P.Stress granules,P-bodies and cancer[J].Biochimicaet Biophyssica Acta:Gene Regulatory Mechanisms,2015,1849(7):861-870.
- [110] LAPLANTE M,SABATINI D M.mTOR signaling in growth control and disease[J].Cell,2012,149(2):274-293.
- [111] MAHBOUBI H,KODIHA M,STOCHAJ U.Auto-mated detection and quantification of granular cell compartments[J].Microsc Microanal,2013,19(3):617-628.
- [112] SOMASEKHARAN S P,EL-NAGGAR A,LEPRIVIER G,et al.YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1[J].J Cell Biol,2015,208(7):913-929.
- [113] POBLETE-DURÁN N,PRADES-PÉREZ Y,VERA-OTAROLA J,et al.Who regulates whom? An overview of RNA granules and viral infections[J].Viruses,2016,8(7):180.
- [114] WHITE J P,CARDENAS A M,MARISSEN W E,et al.Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase[J].Cell Host & Microbe,2007,2(5):295-305.