(厦门大学生命科学学院,细胞应激生物学国家重点实验室,福建 厦门 361102)
(State Key Laboratory of Cellular Stress Biology,School of Life Sciences,Xiamen University,Xiamen 361102,China)
dendritic cell; antigen-presentation; cancer immunotherapy; tumor microenvironment
DOI: 10.6043/j.issn.0438-0479.202111022
备注
树突状细胞(dendritic cell,DC)作为重要的抗原递呈细胞,在调节先天性免疫和获得性免疫反应中都扮演着重要的角色.近年来,关于调节DC的功能以促进抗肿瘤免疫得到了越来越多的关注.在肿瘤微环境(tumor microenvironment,TME)中,通过DC加工和递呈肿瘤相关抗原(tumor-associated antigens,TAAs)来启动抗原特异性的T细胞应答; 反之,肿瘤细胞通过释放代谢产物、细胞因子等调节TME来抑制DC的招募和功能.肿瘤组织中存在着不同亚群的DC,其功能各不相同.深入了解DC亚群在TME中的多样性和功能,可以有效提高DC的抗肿瘤免疫疗法.本文重点论述不同DC亚群在调节抗肿瘤免疫反应中的功能,以及基于DC免疫疗法的研究进展,为将来的基础和临床研究提供新的思路和策略.
Background: Dendritic cells (DCs) are a diverse group of professional antigen-presenting cells that plays an essential role in initiating innate and adaptive immune responses. Derived from a hematopoietic lineage, DCs consist of developmentally and phenotypically distinct subpopulations. Among these subsets of DCs, conventional DCs (cDCs) are specialized in antigen presentation for T cell activation. cDCs comprise two main subsets, the type 1 cDCs (cDC1s) and type 2 cDCs (cDC2s). cDC1s are specialized in presenting antigens to CD8+ T cells, whereas cDC2s are efficient in priming CD4+ T cells. In addition to cDC1s, plasmacytoid DCs develop from both common DC precursors and lymphoid progenitors, which can rapidly produce type Ⅰ interferons upon virus infection. Moreover, circulating Ly6Chigh monocytes can be differentiated into monocyte-derived DCs under inflammatory conditions and possess pro-inflammatory functions. Although DCs constitute a rare population of immune cells within tumor site and lymphoid organs, they are crucial for the mediation of antigen-specific immunity. DCs regulate immune responses or tolerance by presenting antigens to T cells, providing immunomodulatory signals and cytokine secretion.
Progress: DCs serve as professional antigen-presenting cells and play essential roles in the regulation of innate and adaptive immune responses. In the tumor microenvironment, DCs process and present tumor-associated antigens to initiate antigen-specific T cell responses. On the contrary, tumor cells produce immunosuppressive factors that can inhibit DCs’ infiltration and dampen their antitumor immunity. Herein, there is growing interest in modulating DCs’ function to induce efficient antitumor immunity. A better understanding of the diversity and functions of DCs’ subpopulations and the underlying mechanisms that regulate DCs’ function could lead to improved cancer immunotherapy. DCs have been applied to tumor immunotherapy in various kinds of preclinical or clinical trials, and DCs’ vaccination presents great potential for cancer immunotherapy. It has been shown that DCs’ vaccination is able to effectively prevent metastasis and relapse after surgical treatment of different cancers. Therefore, DCs-based immunotherapies can provide novel strategies for future clinical treatment against cancer.
Perspective: As the most potent antigen-presenting cells, DCs are able to activate naive T cells and induce antigen-specific immune responses in cancer. However, DCs are found to be suppressed or dysfunctional in the tumor microenvironment. A better understanding of how DCs are regulated in these scenarios may promote therapeutic breakthrough in clinical trials. The important question raised here is how the different subpopulations of DCs may lead to unique immune responses in the tumor microenvironment. As discussed in this review, the cDC1s are essential to induce the cross-presentation and cancer-controlling cytotoxic T cell responses, accompanied with increased survival rate in specific cancer types. On the other hand, the cDC2s are crucial for the induction of CD4+ T cell antitumor immunity. It has been well documented that DCs can enhance the efficacy of antitumor immunotherapies, but the optimal vaccination development requires further knowledge of DCs’ characters and functions. Approaches to develop the optimal DCs’ vaccination need to consider the following aspects, such as combination of conjunction with neoantigens, usage of adjuvants and enhancement of endogenous DCs’ costimulatory function. More importantly, precise and specific DCs’ targeting strategy leads to enhanced efficacy of these strategies.
引言
树突状细胞(dendritic cell,DC)作为一种抗原递呈细胞,是免疫系统的核心参与者,也是先天免疫和获得性免疫反应的重要调节剂,能够诱导免疫系统对病原体的免疫反应,也能对无害抗原建立免疫耐受,同时维持两者之间的平衡,刺激或抑制T细胞反应.DC遍布于全身各处,由于位置、固有层免疫位点和组织特异性的不同,又特化为不同的类群.因此,明确DC亚群的特异性和环境因素对不同亚群DC功能的影响,是启动适应性免疫反应治疗肿瘤的关键.
DC不仅是免疫器官里罕见的细胞群,也是肿瘤组织中少见的免疫细胞群,主要通过摄取并呈递抗原给T细胞、细胞之间的直接接触和提供细胞因子来调节免疫信号.环境因素对DC功能的调节至关重要,可通过细胞表面和细胞内的细胞因子受体、病原体相关分子模式和损伤相关分子模式来调控抗原特异性免疫和耐受性启动[1].因此,深入研究DC在肿瘤免疫学中的主要功能,对于有效启动抗肿瘤免疫以及靶向DC在癌症患者中的治疗潜力具有重大意义[2].
1 DC的分类
起初根据发育起源不同,将DC分为淋巴系和骨髓系[2],后来与功能相关的一种基于个体发育的分类系统将DC分为经典DC(conventional DC,cDC)、单核细胞衍生的DC(monocyte-derived DC,moDC)、浆细胞样DC(plasmacytoid DC,pDC)和朗格汉斯细胞(Langerhans cell,LC)[3].最近发现的一组定义谱系个体发育的特定转录因子可以明确区分单核细胞和DC[4],以及cDC和其他不同的DC亚群,DC亚群的特征标志基因和蛋白质见表1.
1.1 cDCcDC特异性表达转录因子Zbtb46,是稳态条件下最为丰富的DC类群,遍布于体内所有组织[4].根据其最初所处的位置不同,可将cDC分为淋巴组织驻留DC(residence DC,resDC)和迁移性DC(migratory DC,migDC)两大类.resDC主要存在于脾脏、淋巴结或派尔氏淋巴结中,不断从血液进入淋巴结,并通过
表1 已有研究确定的小鼠DC亚群的特征标志基因和蛋白质[5-7]
Tab.1 Characteristic gene and protein markers of described murine DC subsets as determined from published resources[5-7]淋巴引流或从其他细胞转移获取抗原,并且可以将这些获得的抗原以抗原肽的形式呈递至CD4+和CD8+ T细胞并启动其活化.migDC起初存在于皮肤、肺或固有层等一些实质组织中,能够在稳态条件或C-C基序趋化因子受体7(C-C motif chemokine receptor 7,CCR7)依赖的炎症诱导激活时,自发迁移到局部的淋巴器官中,与初始T细胞相互作用[8].因此,淋巴组织中既有resDC,也有实质组织来源的migDC,而根据细胞表面分子主要组织相容性复合体(major histocompatibility complex,MHC)Ⅱ和CD11c的表达水平,可将两者区分开[5].其中,resDC高表达CD11c和中度表达MHCⅡ[9]; 而migDC高表达MHCⅡ和CCR7,中度表达CD11c[10].在炎症发生时,resDC在炎性因子的刺激下,也会高表达MHCⅡ和CCR7,因此仍需进一步确定resDC和migDC特异性稳定表达的表面标志物.由于表面标志物的局限性,研究者利用RNASeq 技术对resDC和migDC不同的转录组进行分析[6].研究表明,migDC高表达Ccr7和Fscn1等转录因子,而resDC却不表达; H2dma和H2dmab等一些MHCⅡ转录本在migDC中低表达,这与成熟migDC处理过量抗原能力降低一致[6-7].
cDC根据个体发育和转录组不同,又可分为1型(cDC1)和2型(cDC2)[7,11].cDC1主要由Ly6C-的DC前体细胞依赖于Batf3和Irf8发育而来[12-15],其表面的X-C基序趋化因子受体(X-C motif chemokine receptor 1, XCR1)主要在cDC1特异性高表达[16].cDC1的主要功能是将抗原提呈给CD8+T细胞和启动Th1(T helper 1)细胞免疫应答,产生白细胞介素-12(interleukin-12,IL-12)以及特异性表达Toll样受体3(Toll-like receptor 3,TLR3)[17-19].而cDC2优先从Ly6C+的DC前体细胞发育而来[15],其发育主要受到高表达的转录因子干扰素调节因子4(interferon regulatory factor 4,IRF4)调控[20-22],此外cDC2特异性高表达SIRPα[23].cDC2主要作用于CD4+T细胞,调控Th2、Th17细胞和调节性T细胞(regulatory T cell,Treg)介导的免疫应答.
DC前体细胞遍布于淋巴组织和外周组织中[24-25],而cDC1和cDC2前体细胞表面趋化因子受体的差异表达决定了它们不同的分布模式.cDC1前体细胞高度表达C-X-C基序趋化因子受体3(C-X-C motif chemokine receptor 3,CXCR3),其配体是干扰素γ(interferon γ,IFN-γ)诱导的C-X-C基序趋化因子配体9(C-X-C motif chemokine ligand 9,CXCL9)、CXCL10、CXCL11,因此cDC1更容易被募集到IFN-γ介导的炎症部位[26].此外,cDC1和cDC2的相对占比在不同组织间有明显差异[27],例如cDC1更多分布在肺组织中,而在皮肤中较少[5].
1.2 moDC除cDC外,机体内还有许多其他类群的DC.例如在炎症发生时,高表达Ly6C和CD11b的单核细胞会被招募到炎症或感染部位,分化为表达CD11c的moDC,moDC具有更强的抗原提呈能力[28-29].在体外,单核细胞在粒细胞-巨噬细胞集落刺激因子(granulocyte-macrophage colony-stimulating factor,GM-CSF)和IL-4的刺激下,以依赖IRF4的方式分化为moDC[30-31],而在Irf4基因缺失时,GM-CSF和IL-4刺激单核细胞可分化为巨噬细胞[30],这一现象表明IRF4是促使moDC分化的重要调节因子.在体内,moDC存在于炎症和感染组织中[32-33],因此一些炎症相关信号转导可能也参与调控单核细胞向moDC的分化[34].
1.3 pDCpDC也是DC的一个亚群,虽然和cDC拥有共同的祖细胞,但是它在个体发育和功能上与cDC不同[35-37].MHCⅡ和CD11c中度表达的pDC不会在淋巴结中发生迁移,它既可以来源于淋巴也可来源于骨髓[38-39],可通过IRF7、SiglecH以及CD317的高表达来识别pDC[40].在功能上,cDC调控未成熟T细胞的活化和分化,而pDC产生大量IFN-Ⅰ和促炎细胞因子以响应病原体刺激; 而骨髓衍生的pDC能够将抗原呈递给T细胞,但它们在调节CD4+T细胞应答方面的作用仍有待确定[36-37].
1.4 LCLC是骨髓衍生的DC,与巨噬细胞拥有一些共同特性,通常位于表皮的基底层,与周围角质层细胞形成密切的细胞接触,为其在表皮的存活提供必要条件[41-42].LC可通过细胞表面表达的CD207、E-钙黏蛋白、CD326以及低表达CD11b来识别; 同时由于它们不表达XCR1和CD103,所以可与皮肤中的cDC1区分开[5].在抗原入侵时,LC会上调MHCⅡ和共刺激分子的表达并迁移至局部淋巴结,它们摄取抗原并呈递给T细胞,从而引发全身性免疫应答.在稳态条件下,LC能够在皮肤中维持数月或数年,但稳态条件下的LC更新是否可能由表皮LC或皮肤中存在的不同LC前体群介导,仍需进一步研究[43].
2 DC的功能
2.1 摄取抗原DC作为免疫防御的第一道防线,不断从局部组织中摄取抗原,并在处理后分别呈递给CD4+T细胞或CD8+T细胞[44-45].对于外源性抗原,根据屏障部位DC亚群的活化状态不同,能够通过细胞的吞噬作用、受体介导的内吞作用或者微胞饮作用进行摄取[46-51].例如:在表皮或肠上皮等屏障区域,LC和CX3CR1+的巨噬细胞通过紧密连接将DC固定在角质化上皮层或肠腔中[43,52-54],以CXCR4依赖性方式通过真皮转运至皮肤淋巴组织中[55].由于真皮cDC能以更快的速度迁移到淋巴结,所以这种迁移会显著提高表皮源性抗原到达淋巴结的效率[56-57].肠道中的DC可通过跨细胞转运摄取可溶性抗原[58],也可通过M细胞或形成杯状细胞相关通道摄取颗粒抗原[59-60].皮肤中的cDC2可以通过毛囊直接接触表皮抗原[61].在小鼠模型中利用荧光标记的微粒抗原来追踪皮肤中发生迁移的DC,观测到这群细胞将荧光抗原运输到淋巴结,并且在该免疫点伴随着抗原特异性CD4+T细胞的浸润,这一现象表明cDC2更倾向于皮肤中的抗原摄取[62-65].
同时cDC2在肿瘤抗原的摄取、转运至淋巴结以及提呈给T细胞等方面也起到重要作用.cDC2分布于淋巴结内的包膜下窦附近,这是摄取淋巴结携带抗原的最佳位置[65-66].而cDC1位于淋巴结旁皮质的深处[66],主要通过受体(如Clec9A、DEC205、Axl和TIM3)有效摄取细胞相关抗原和死亡细胞[67].cDC1优先通过交叉呈递途径加工处理细胞相关抗原,在MHCⅠ上呈递至CD8+T细胞,这对于抗病毒和抗肿瘤免疫至关重要[13,67].已有研究表明,靶向Clec9A和DEC205的单克隆抗体,能够增强疫苗接种中的抗原摄取和交叉呈递[68-70].
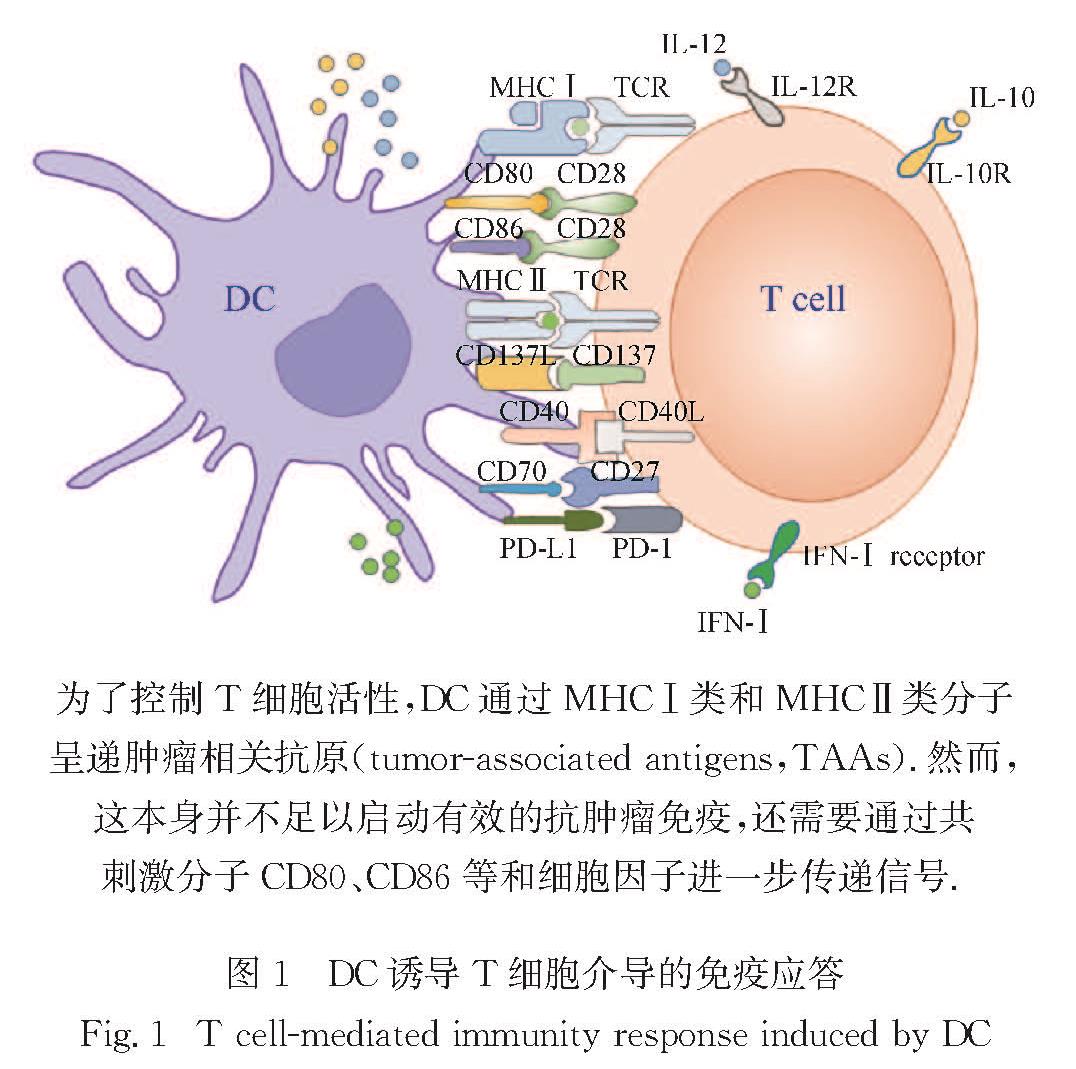
2.2 启动T细胞DC是体内主要的抗原呈递细胞(antigen presenting cell,APC),通过MHC-T细胞受体(T cell receptor,TCR)和共刺激信号传导的协同作用,引发初始T细胞的抗原特异性激活和增殖,DC诱导的T细胞免疫应答如图1所示.cDC2在加工处理抗原后通过MHCⅡ呈递抗原肽给CD4+T细胞,并促使其增殖[68,71].有研究表明,cDC2中Irf4的表达与MHCⅡ表达增强有关[71],因此Irf4的表达被认为是cDC2特化的原因.除促进CD4+T细胞的活化和增殖外,在抗原呈递过程中DC和T细胞之间的通讯也能促使Th1细胞分化,例如,共刺激信号、MHCⅡ-TCR相互作用的强度和持续时间等都可调节Th1细胞分化[72].来自病原体、细胞基质以及周围免疫细胞的不同信号传导促使DC启动不同的响应,这确保了效应Th1细胞的适当激活[73].
有研究表明,在DC中条件性缺失Irf8的小鼠体内,无论是在稳态下还是炎症反应时,都缺失了cDC1,同时Th1细胞明显减少[74-75].这一现象表明,cDC1在诱导Th1细胞的分化上起重要作用.此外有研究表明,来自DC周围细胞的IFN-γ能够诱导DC分泌更多的IL-12[76-77],也能促进Cxcl9和Cxcl10的转录[78].DC产生的这些因子与IL-27共同促进Th1细胞的分化.其他基质细胞也能通过模式识别受体(pattern recognition receptor,PRR)的信号传导,例如TLR3、TLR9、TLR11和TLR12可诱导DC上调IL-12的表达[79].而蠕虫的产物也可通过PRR信号传导以及周围细胞产生的IFN-Ⅰ和胸腺基质淋巴生成素(thymic stromal lymphopoietin,TSLP)调节DC以促进体内Th2细胞分化[80-81].由TLR2和Dectin-1参与的PRR信号转导促使DC产生IL-6和IL-23,二者与整合素αvβ8介导的转化生长因子β(transforming growth factor β,TGFβ)共同促进Th17细胞的分化[82-84].而成纤维细胞来源的前列腺素和神经元来源的降钙素也能刺激DC分泌更多的IL-23,加速Th17细胞的分化[85-87].
DC也可促进Treg细胞的产生.在稳态条件下视黄醇可以调控DC成为耐受状态,此时DC产生B细胞和T细胞衰减因子及TGFβ,这些因子在没有免疫刺激信号时促进Treg细胞的产生[88-89].DC可以通过PRR和IFN-Ⅰ分泌更多的IL-6,而IL-6可参与滤泡辅助性T(T follicular helper,Tfh)细胞的分化[90].IL-2会抑制IL-6的表达,而DC会通过表达CD25以抑制IL-2的功能,进一步促使Tfh分化[91-93].同时,cDC1的交叉提呈被认为是启动细胞毒性T淋巴细胞(cytotoxic T lymphocyte,CTL)反应的关键[13,68].有文献报道,TLR7在体内的激活增强了固有层cDC1向肠系膜淋巴结的迁移,cDC1在肠系膜淋巴结中,将抗原提呈给表达IFN-γ的CD8+效应T细胞[94].
2.3 免疫耐受在稳态下,cDC以耐受的方式递呈某些外来抗原,促使CD4+T细胞分化为Foxp3+Treg细胞.特别是在肠道中,从淋巴组织中迁移过来的cDC将这种无害抗原递呈给肠系膜淋巴结中的未成熟T细胞.
脾脏和淋巴结中的cDC高表达乙醛脱氢酶,这些酶能够催化维生素A代谢产物视黄醛转化为维甲酸(retinoic acid,RA)[89,95].在抗原呈递后,RA可诱导未成熟的T细胞分化为Foxp3+Treg细胞[89,95],而表达乙醛脱氢酶的肠道cDC也以RA依赖性方式诱导发育的T细胞表达高水平的CCR9和整合素α4β7.该作用机制可能也是肠道免疫系统维持对食物源性抗原耐受性的主要机制之一,被称为口服耐受[96-97].有研究表明,cDC缺失或阻断cDC向肠系膜淋巴结的迁移,会导致口服耐受失败[58,98].另有研究表明,结肠淋巴中cDC的乙醛脱氢酶表达相对较低,这一现象与结肠淋巴和肠系膜淋巴结中RA的表达水平较低相关[99].上述研究结果意味着,根据cDC功能专一性分离的解剖学基础,肠道近端和相关淋巴迁移更容易形成免疫耐受,而更多远端部位可能有利于诱导免疫应答[100].然而在结直肠内注射可溶性抗原后,结直肠中迁移的cDC可诱导Foxp3+T细胞产生免疫反应和耐受[100].因此进一步阐明结直肠cDC诱导免疫耐受的机制,以及其与脾脏淋巴组织中cDC的诱导机制的差异有重要的意义.此外,根据其解剖位置,cDC暴露于不同来源的抗原,脾脏淋巴组织中cDC可能主要负责食物源性的可溶性抗原的处理和呈递,而结直肠淋巴组织中cDC可能会遇到来自肠道微生物群的更高水平的抗原[101].
在稳态下,肠道中的cDC具有一系列适应性,使它们能够在发育中的T细胞上留下肠道归巢和耐受性特征.有研究表明,特异性缺失CD11c+细胞上的整合素αVβ8会导致Foxp3+T细胞的形成显著减少,这可能和cDC能够表达整合素αVβ8并产生耐受性细胞因子TGFβ有关[102].也有研究表明,CD11c+细胞分泌的IL-33能够促进Foxp3+T细胞的分化,这可能是cDC诱导免疫耐受和维持肠道内稳态的另一种机制[103].而cDC这些耐受表现可能是组织调节导致的,也可能是组织微环境中信号的积累所致[96].当阻断cDC中RA或TGFβ的信号传导后,耐受性cDC2的形成明显减少,这一现象也表明cDC在免疫耐受的调节中起重要作用.此外,上皮细胞产生的TSLP、芳香烃受体配体、前列腺素和血管活性肠肽等其他局部因子均可对cDC产生调节作用[104-106].特别是在肠道中,细菌代谢产物短链脂肪酸也有助于维持耐受性cDC的功能[107].同时肠系膜淋巴结微环境中的基质成分也可能影响迁移性cDC的耐受功能[108-109].
3 DC在肿瘤免疫中的作用
肿瘤微环境(tumor microenvironment,TME)中常见的宿主免疫细胞有单核/巨噬细胞、DC、中性粒细胞、自然杀伤(natural killer,NK)细胞和T细胞.这些细胞在抗肿瘤免疫中有不同的功能,其中,DC作为抗原特异性免疫和耐受的启动中心,在诱导抗肿瘤免疫中有着独特的作用[110].本文将讨论DC如何在肿瘤环境中发挥抗肿瘤免疫的作用,以及肿瘤本身对DC的募集和功能的影响.
3.1 DC促进抗肿瘤免疫在肿瘤免疫中,DC通过识别肿瘤细胞特异性抗原,将其信号呈递给具有杀伤效应的T细胞实现监测、杀灭肿瘤的功能.首先,DC将TAAs递呈给T细胞,诱导大量的效应T细胞发挥抗肿瘤作用.cDC1能高效加工并通过MHC Ⅰ类分子交叉递呈外源抗原来激活CD8+T细胞,并启动Th1细胞应答能力,在抗肿瘤免疫中有着非常重要的作用[111].而cDC2和moDC也可以向T细胞呈递抗原,cDC2在抗肿瘤CD4+T细胞免疫应答的启动中有着重要的作用[15,112].根据环境的不同,人的cDC2可以诱导Th细胞不同亚群分化并激活CD8+T细胞介导的免疫应答[111,113-114].例如,IRF4依赖的CD24+CD11b+ cDC2可以分泌IL-23α并调控黏膜IL-17介导的免疫应答[22],还可以通过调节细胞因子IL-10和IL-33的产生,特异性地促进Th2细胞分化[115].
一旦感知到外界抗原,DC在成熟的过程中还会上调共刺激分子来提供T细胞活化需要的信号.例如:DC表达的CD80和CD86分别通过与CD28或CTL抗原4(CTL antigen 4,CTLA4)相互作用调控T细胞的激活或抑制[116].CD4+和CD8+ T细胞上均表达CD137(又称4-1BB),可与DC等APC上表达的CD137L(又称4-1BBL)结合,促进CD4+和CD8+ T细胞的活化和存活[117].DC和巨噬细胞上的OX40L也有助于T细胞存活,从而有利于抗肿瘤免疫[118].此外,DC表面还会表达CD40,CD40与CD40L相互作用对CD4+T细胞的功能至关重要.这些分子的相互作用会导致相关T细胞的完全激活,而缺乏这种重要的相互作用则导致CD4+T细胞的活化大幅减少[119].此外,DC上表达的共刺激分子CD70的受体为T细胞上表达的CD27.通过靶向CD27的激动剂抗体与抗程序性死亡受体1/配体1(programmed cell death protein 1/ligand 1,PD-1/L1)的协同作用,可以增强CD8+ T细胞增殖和抗肿瘤效应[120].而DC上表达的可诱导共刺激分子配体(inducible costimulatory ligand,ICOSL)可以通过与ICOS结合而活化T淋巴细胞,并刺激其细胞因子的分泌[121].
此外,DC还可以通过分泌趋化因子和细胞因子等来影响肿瘤的进程.pDC上的TLR7和TLR9被激活后,可以大量分泌IFN-Ⅰ(即IFN-α/β)[35,122].IFN-Ⅰ既可以抑制肿瘤细胞的增殖,又可以活化免疫系统进而发挥抗肿瘤效应[123],在临床上常用于肿瘤治疗[124-125],许多传统化疗药物、靶向抗癌药物、免疫佐剂只有在完整的IFN-Ⅰ信号存在时才完全有效.IL-12家族包括多种细胞因子,如IL-12、IL-23、IL-27、IL-15和IL-18,它们可以促进NK细胞的激活并发挥效应功能[126].cDC1和cDC2均可在TLR刺激后产生IL-12,且人类肿瘤中IL-12的水平也与cDC1浸润增加有关[77].在浆细胞瘤小鼠模型中,使用表达IL-27的重组腺相关病毒(adeno-associated virus,AAV)可显著抑制肿瘤生长并增强肿瘤中的T细胞反应[127].DC还可以在TME中产生募集T细胞的趋化因子,例如:cDC1是TME中CXCL9和CXCL10的主要分泌细胞,CXCL9和CXCL10可以促进CD8+ T细胞向TME浸润[128].
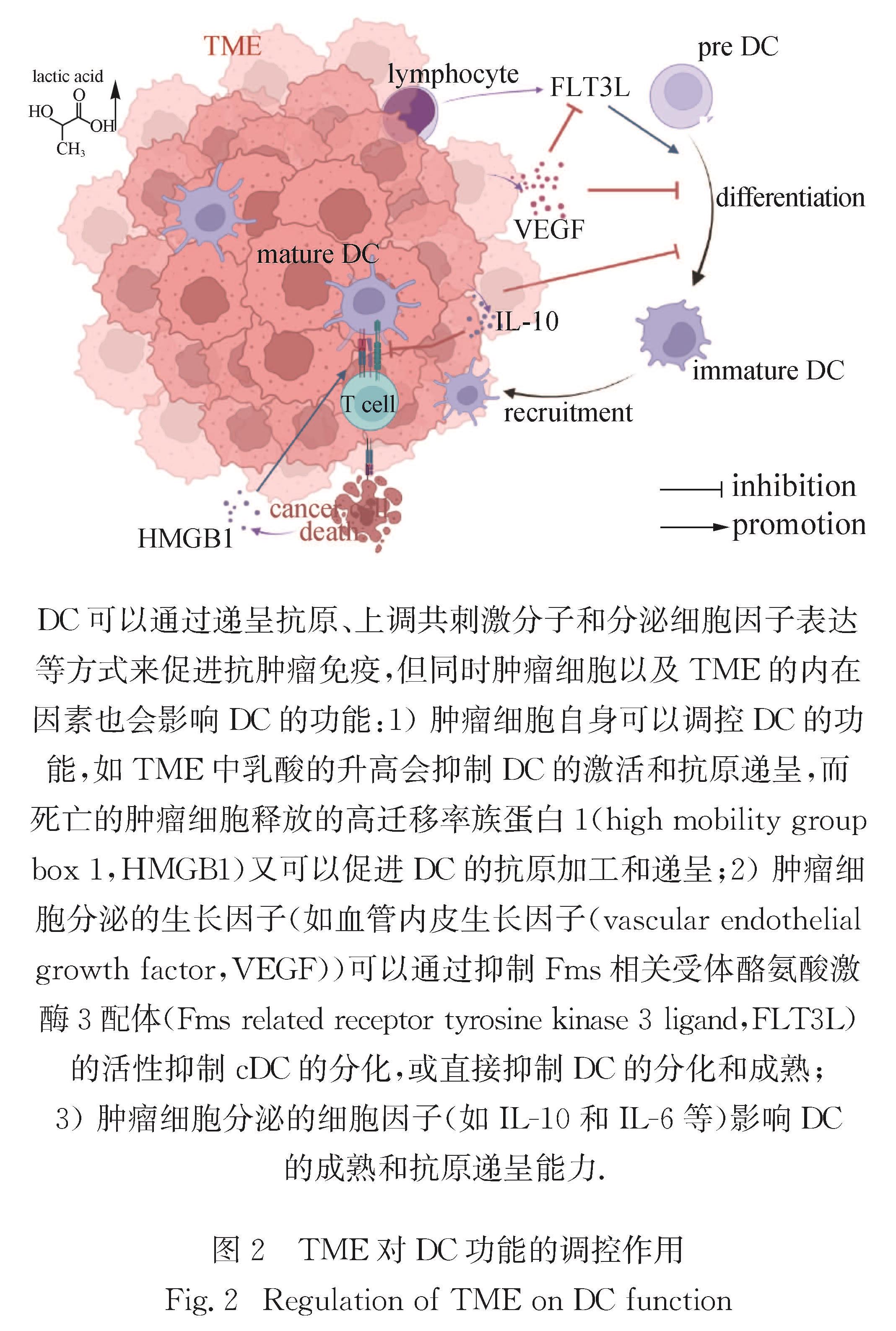
3.2 TME对DC的影响由于免疫监测的某些缺陷,肿瘤细胞会逐渐获得各种机制以逃避免疫细胞的识别和清除,有利于肿瘤进一步生存和进展.例如,肿瘤细胞可以通过自身释放的物质和一些生长因子、细胞因子等来影响或限制DC的功能,常见的一些影响途径如图2所示.
肿瘤细胞的内在因子和TME中的代谢物会影响DC的功能.在人类和小鼠中,具有活性β-连环蛋白的肿瘤会减少C-C基序趋化因子配体4的表达,导致cDC1浸润降低并加速肿瘤的生长[129].肿瘤来源的一些代谢产物(如乳酸),是调节TME中DC激活和抗原递呈的重要因素,也可能是肿瘤逃逸的关键机制之一,而通过阻断乳酸的生成可以恢复TME中DC的正常表型[130].肿瘤来源的神经节苷脂和前列腺素也会抑制cDC的成熟和存活以及moDC的分化[131].TME中过度衍生的缺氧诱导因子α会抑制DC对CD8+T细胞的刺激能力[132]; 缺氧也可造成腺苷的累积,腺苷可通过其受体抑制DC的功能,促进TME中免疫耐受的形成[133].此外,死亡的肿瘤细胞释放的免疫原信号可以促使DC摄取、加工和提呈抗原,例如营养缺乏或先天免疫反应导致死亡的肿瘤细胞释放HMGB1,HMGB1可以通过TLR4被人类和小鼠DC感知,从而促进从死亡癌细胞中摄取的TAAs的高效加工和交叉提呈[134].在结直肠癌模型中,发现TME中的DC通过去小分子泛素相关修饰物化酶3感应活性氧并增强胞质DNA诱导的干扰素基因刺激因子(simulator of interferon genes,STING)信号激活,从而促进DC的抗肿瘤功能[135].
TME中的DC关键代谢通路会被改变,进而对其介导的肿瘤免疫产生影响,这也是肿瘤免疫逃逸的重要因素.由肝癌细胞分泌的一种蛋白质——甲胎蛋白,会抑制DC的脂肪酸合成和线粒体代谢,导致DC刺激抗原特异性效应因子的能力受损[136].来自肿瘤的DC会积累氧化脂质,促进肿瘤的进展[137].小鼠肿瘤中的DC脂质代谢异常,导致DC中甘油三酯累积,而富含脂质的DC处理抗原的能力降低; 用乙酰辅酶A羧化酶抑制DC的脂质丰度,可恢复DC的功能活性,并显著增强肿瘤疫苗的效果[138].
肿瘤细胞也可以通过分泌一些生长因子,来限制DC的发育和成熟.VEGF是大多数肿瘤分泌的肝素结合蛋白,负责肿瘤新生血管的形成:它一方面可以通过结合和激活两种酪氨酸激酶受体VEGFR-1和VEGFR-2来抑制DC的分化和成熟; 另一方面,由肿瘤浸润性淋巴细胞产生的FLT3L对cDC的发育和增殖至关重要,并会促进其生存[139].然而,VEGF在体外可抑制FLT3L的活性,负调控cDC的分化[140].VEGF还可以通过阻断造血干细胞中核因子κB的活化抑制体内外DC的成熟[141].肿瘤细胞还可以通过产生一些细胞因子或趋化因子影响DC的功能,例如:肿瘤细胞可以通过产生促炎因子IL-6抑制cDC和moDC的分化[131]; 在黑色素瘤、多发性骨髓瘤和肺癌等肿瘤中,肿瘤细胞可以通过释放抑炎因子IL-10抑制DC的成熟及其活化T细胞的能力[142]; Terra等[143]利用pDC缺失的小鼠模型,发现肿瘤细胞通过TGF-β抑制pDC激活和炎性因子分泌功能; Combes等[144]发现TGF-β处理后的pDC或从人乳腺肿瘤中分离的pDC,都会持续地表达溶酶体相关膜糖蛋白5从而抑制pDC分泌IFN-Ⅰ.
4 DC在肿瘤治疗中的应用
5 展 望
DC作为重要的抗原递呈细胞,在活化T细胞和抗肿瘤免疫反应中起重要作用.然而,在TME中DC常处于免疫抑制或免疫耐受的状态.目前,不同DC亚群在肿瘤发生发展过程中的功能引起了越来越多的关注.深入了解不同DC细胞亚群在肿瘤组织中的作用及其免疫调节机制对临床治疗,具有重要意义.在TME中,cDC1能诱导杀伤性T细胞的抗肿瘤免疫反应并提高癌症患者的生存率,cDC2能够诱导CD4+ T细胞的抗肿瘤免疫功能.深入了解TME抑制DC募集、迁移以及成熟的机制,对于增强已建立的癌症疗法的效果、改善癌症患者预后有重要意义.目前还有以下问题亟待进一步的研究:在联合免疫治疗中,cDC1调节抗肿瘤免疫的具体机制是什么?cDC1除了招募并激活CD8+ T细胞,是否也会增强肿瘤中NK细胞的功能?反之,NK细胞是否能活化并增强cDC1的功能?是否能将cDC1在肿瘤组织中的数量作为癌症治疗预后的一项指标?在TME中调节DC功能的具体因子和机制是什么?通过对DC亚群特定功能的进一步探究和了解,精准靶向DC、增强DC功能并结合现有的免疫疗法、放疗、化疗等癌症疗法,对于改善癌症患者治疗效果具有重要指导意义.
4.1 DC在各种肿瘤治疗手段中的作用恶性肿瘤的三大传统治疗手段为手术、放疗和化疗,而DC对放疗和化疗的反应性都有重要的影响.在一些新型的肿瘤治疗手段(如免疫检查点治疗和小分子抑制剂治疗等)中也发挥着重要的作用.
某些化疗药物和靶向肿瘤的药物,如硼替佐米(bortezomib)、 阿霉素(doxorubicin)、表阿霉素(epirubicin)、柔红霉素(idarubicin)、米托蒽醌(mitoxantrone)和奥沙利铂(oxaliplatin)等,可以触发免疫原性细胞死亡,促进抗肿瘤免疫,而这一过程依赖于DC[145-146].如蒽环类药物诱导的细胞死亡促进moDC向TME募集,moDC又将TAAs交叉呈递给CD8+ T细胞[147].免疫原性细胞死亡后,会将钙网蛋白(calreticulin)暴露于细胞表面,这一信号也会促使肿瘤细胞被DC摄取[148].肿瘤细胞的免疫原性死亡也会导致三磷酸腺苷(adenosine triphosphate,ATP)的释放,从而促进DC募集和IL-1β的产生[149].此外,ATP还可以促进肿瘤细胞释放HMGB1,而HMGB1可以通过TLR4被DC感知,从而促进DC加工并呈递死亡的肿瘤细胞的TAAs[134].但并非所有的化疗药物都是通过诱导免疫原性细胞死亡而发挥作用的.
放疗的作用一般是直接杀死肿瘤细胞,但放疗同样可以通过调控DC功能来影响肿瘤治疗的效果.例如,放疗后肿瘤细胞释放的细胞质DNA可以通过环磷酸鸟苷-腺苷合成酶(cyclic guanosine monophosphate adenosine synthase,GAS)-STING信号通路来诱导DC产生IFN-Ⅰ,从而有助于抗肿瘤免疫[150].
DC与抗PD1治疗的反应性相关,靶向PD1、PDL1或CD137的治疗手段可以放大由DC启动的抗肿瘤免疫反应.有研究表明,缺乏cDC1的Batf3基因缺陷小鼠在肿瘤移植模型中对抗PD1、抗PDL1或抗CD137治疗均不应答[151]; 而DC通过囊泡运输蛋白SEC22B介导的TAAs交叉呈递对PD1阻断治疗是必需的[152].通过靶向IFN-Ⅰ激活cDC1也可以改善抗PDL1的疗效[153].在胰腺癌模型中,CCR4转导的CD8+ T细胞与DC间相互作用的能力增强,从而产生更强的抗肿瘤活性[154].而当前临床上常用的方法,还有将TAAs致敏后的DC联合细胞因子诱导的杀伤细胞(cytokine-induced killer cell,CIK)共培养后回输至患者体内,共同行使杀伤肿瘤细胞的作用,称作DC-CIK免疫疗法[155].
小分子抑制剂通常靶向肿瘤中关键致癌信号通路,如信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)、有丝分裂原活化蛋白激酶、雷帕霉素靶蛋白通路,但同时也会影响免疫细胞.比如STAT3的激活会产生一种促进肿瘤生长的炎症,并抑制DC介导的抗肿瘤免疫反应,STAT3的抑制剂JSI-124就可逆转肿瘤中功能异常的DC[156-157].
4.2 DC肿瘤疫苗及发展前景除在各种肿瘤治疗中发挥作用外,基于DC本身的功能也有相应的抗肿瘤研究策略,例如DC肿瘤疫苗.由于DC的强大抗原递呈活性和T细胞活化特性,以及体外分离培养DC细胞技术的发展,利用 DC疫苗治疗各类肿瘤的临床研究逐渐受到广泛关注.与预防性疫苗不同,DC疫苗主要作为治疗性疫苗使用.DC肿瘤疫苗是将肿瘤细胞的DNA、RNA、肿瘤细胞裂解物、肿瘤抗原蛋白或多肽等致敏DC再回输入患者体内,使T细胞重新识别肿瘤抗原,激活T细胞对肿瘤的免疫反应,从而达到治疗肿瘤的目的.越来越多的临床试验表明,通过给患者输入负载肿瘤抗原的自身DC,可以诱导患者机体产生特异性持久的抗肿瘤免疫应答,提高患者生存率.
4.2.1 DC肿瘤疫苗的制备目前DC疫苗的制备主要有两种方法:一是肿瘤抗原负载.从患者的外周血中分离出单核细胞,将分离的单核细胞在GM-CSF和IL-4中培养生成未成熟的DC; 再利用各种细胞因子混合物使DC成熟或激活DC,并通过电融合技术将肿瘤细胞裂解物载入DC; 成熟的DC随后通过皮下或皮内注射回患者体内.二是病毒载体携带抗原基因转染DC.使用病毒载体携带抗原基因,可以使基因在DC内稳定表达,从而使DC获得持续大量的抗原肽刺激,且在此过程中被诱导成熟.目前常用的病毒载体有AAV、逆转录病毒和腺病毒等.有研究表明,采用AAV使DC负载癌胚抗原诱导CTL,用于癌胚抗原高表达的结直肠癌的治疗,在结直肠癌的细胞株、移植瘤模型中均验证了这种诱导特异性CTL的治疗作用[158].Mazzolini等[159]在进行的一项临床试验中,将携带IL-12编码基因的腺病毒感染DC,瘤内注射治疗转移性胃肠癌,发现15例患者的血清中IFN-γ和IL-6的浓度升高,5例患者外周血中NK细胞活性升高,且细胞免疫治疗后的患者耐受性良好.
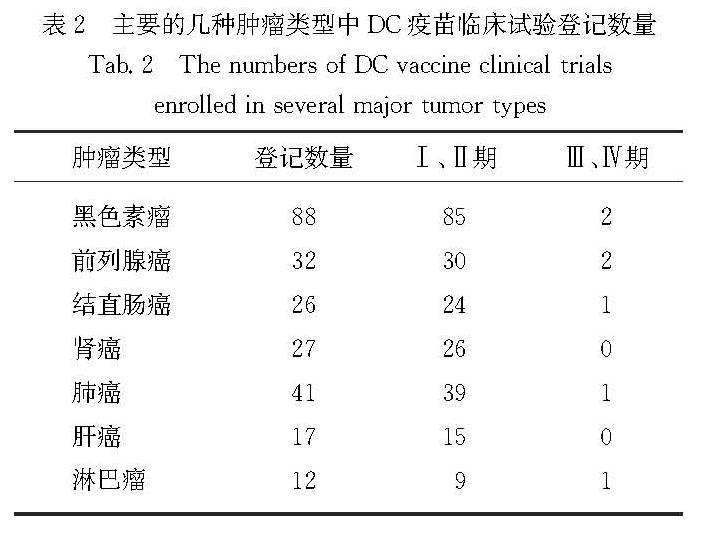
4.2.2 DC疫苗的进展与发展前景Sipuleucel-T是美国食品药物管理局(Food and Drug Administration,FDA)批准的首个用于治疗的DC肿瘤疫苗[160],也是目前为止唯一获得美国FDA批准使用的癌症疫苗.它由与GM-CSF/前列腺酸性磷酸酶(prostatic acid phosphatase,PAP)构建的融合蛋白PAP-GM-CSF共培养的外周血单核细胞组成,用于治疗转移性前列腺癌.研究表明,Sipuleucel-T可以显著延长患者的生存期,但对病情进展时间没有影响.截至2021年10月,美国国立卫生研究院管理的“ClinicalTrails”临床数据登记平台显示,以“dendritic cell vaccine”为关键词搜索,共有476项DC疫苗相关的临床试验登记,其中主要的几种癌症类型已有多项进入临床试验,如表2所示.
表2 主要的几种肿瘤类型中DC疫苗临床试验登记数量
Tab.2 The numbers of DC vaccine clinical trials enrolled in several major tumor types为探讨自体DC疫苗是否能诱导结直肠癌转移灶切除后患者的抗肿瘤免疫应答,2010年Barth等[161]在进行的一项临床试验中,对接受结直肠癌转移切除术的患者进行结内注射自体肿瘤裂解液致敏的DC疫苗或安慰剂治疗,在很高比例的患者中发现DC疫苗诱导的肿瘤特异性免疫反应.
在黑色素瘤中,DC疫苗开展的临床应用较多.用携带肿瘤坏死因子相关凋亡诱导配体(tumor necrosis factor related apoptosis-inducing ligand,TRAIL)基因的重组腺病毒转染小鼠来源的DC,DC-TRAIL可明显诱导B16凋亡; 将DC-TRAIL注射于B16肿瘤接种部位,在TRAIL有效杀伤肿瘤细胞的同时,DC摄取肿瘤抗原并有效激活抗肿瘤免疫反应,从而实现靶向治疗和免疫治疗的有效结合,可以显著抑制肿瘤生长[162].
在晚期非小细胞肺癌(non-small cell lung cancer, NSCLC)中,DC-CIK免疫治疗联合化疗可以提高NSCLC的疾病控制率,改善患者的免疫功能和生活质量.研究选取患者的外周血细胞,采集后分离外周血单核细胞,诱导培养DC-CIK,在患者进行化疗的同时进行DC-CIK的静脉回输,可以提高晚期NSCLC患者的免疫功能,且不良反应小[155].
对转移性肾癌患者进行的一项临床研究中,通过基因转染术获得成熟的DC疫苗,以及体外细胞培养技术获得CIK,经淋巴引流区及静脉输注方式按疗程回输患者体内,可以不同程度上缓解病情的进展[163]; 2020年Faiena[164]进行了一项Ⅰ期临床试验,利用碳酸酐酶Ⅸ(carbonic anhydrase Ⅸ,CAⅨ)在肾细胞癌患者组织中显著表达的特点,将GM-CSF与CAⅨ融合,利用病毒载体转染DC.早期数据表明,自体未成熟DC加载GM-CSF与CAⅨ后可以安全地用于转移性肾细胞癌患者,且不会引起任何严重的不良反应,也不会引起CAⅨ特异性免疫反应.
总之,DC作为最有效的APC,在抗肿瘤免疫中的作用不容忽视.临床上基于DC的各类肿瘤免疫疗法已显示初步成效,为更精准有效的肿瘤治疗提供了新思路,但DC疫苗目前的安全性和具体的研发策略还有待进一步研究.
- [1] COLLIN M,BIGLEY V.Human dendritic cell subsets:an update[J].Immunology,2018,154(1):3-20.
- [2] KHANNA K M,LEFRANÇOIS L.Geography and plumbing control the T cell response to infection[J].Immunol Cell Biol,2008,86(5):416-422.
- [3] EISENBARTH S C.Dendritic cell subsets in T cell programming:location dictates function[J].Nat Rev Immunol,2019,19(2):89-103.
- [4] SATPATHY A T,WUMESH K C,ALBRING J C,et al.Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages[J].J Exp Med,2012,209(6):1135-1152.
- [5] GUILLIAMS M,DUTERTRE C A,SCOTT C L,et al.Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species[J].Immunity,2016,45(3):669-684.
- [6] MILLER J C,BROWN B D,SHAY T,et al.Deciphering the transcriptional network of the dendritic cell lineage[J].Nat Immunol,2012,13(9):888-899.
- [7] BLECHER-GONEN R,BOST P,HILLIGAN K L,et al.Single-cell analysis of diverse pathogen responses defines a molecular roadmap for generating antigen-specific immunity[J].Cell Syst,2019,8(2):109-121.
- [8] OHL L,MOHAUPT M,CZELOTH N,et al.CCR7 go-verns skin dendritic cell migration under inflammatory and steady-state conditions[J].Immunity,2004,21(2):279-288.
- [9] DILEEPAN T,MALHOTRA D,KOTOV D I,et al.MHC class Ⅱ tetramers engineered for enhanced binding to CD4 improve detection of antigen-specific T cells[J].Nature Biotechnology,2021,39(8):943-948.
- [10] VERMAELEN K Y,CARRO-MUINO I,LAMBRECHT B N,et al.Specific migratory dendritic cells rapidly tran-sport antigen from the airways to the thoracic lymph nodes[J].J Exp Med,2001,193(1):51-60.
- [11] SCOTT C L,SOEN B,MARTENS L,et al.The trans-cription factor Zeb2 regulates development of conventional and plasmacytoid DCs by repressing Id2[J].J Exp Med,2016,213(6):897-911.
- [12] GRAJALES-REYES G E,IWATA A,ALBRING J,et al.Batf3 maintains autoactivation of Irf8 for commitment of a CD8α+ conventional DC clonogenic progenitor[J].Nat Immunol,2015,16(7):708-717.
- [13] HILDNER K,EDELSON B T,PURTHA W E,et al.Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity[J].Science,2008,322(5904):1097-1100.
- [14] SICHIEN D,SCOTT C L,MARTENS L,et al.IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells,respectively[J].Immunity,2016,45(3):626-640.
- [15] SCHLITZER A,SIVAKAMASUNDARI V,CHEN J,et al.Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow[J].Nat Immunol,2015,16(7):718-728.
- [16] BACHEM A,HARTUNG E,GÜTTLER S,et al.Expression of XCR1 characterizes the Batf3-dependent li-neage of dendritic cells capable of antigen cross-presentation[J].Front Immunol,2012,3:214.
- [17] GUILLIAMS M,GINHOUX F,JAKUBZICK C,et al.Dendritic cells,monocytes and macrophages:a unified nomenclature based on ontogeny[J].Nat Rev Immunol,2014,14(8):571-578.
- [18] DORNER B G,DORNER M B,ZHOU X F,et al.Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells[J].Immunity,2009,31(5):823-833.
- [19] REUTER A,PANOZZA S E,MACRI C,et al.Criteria for dendritic cell receptor selection for efficient antibody targeted vaccination[J].J Immunol,2015,194(6):2696-2705.
- [20] BAJAN~A S,ROACH K,TURNER S,et al.IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation[J].J Immunol,2012,189(7):3368-3377.
- [21] PERSSON E K,URONEN-HANSSON H,SEMMRICH M,et al.IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation[J].Immunity,2013,38(5):958-969.
- [22] SCHLITZER A,MCGOVERN N,TEO P,et al.IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine res-ponses[J].Immunity,2013,38(5):970-983.
- [23] BRETON G,ZHENG S W,VALIERIS R,et al.Human dendritic cells(DCs)are derived from distinct circula-ting precursors that are precommitted to become CD1c+ or CD141+ DCs[J].J Exp Med,2016,213(13):2861-2870.
- [24] LIU K,WASKOW C,LIU X T,et al.Origin of dendritic cells in peripheral lymphoid organs of mice[J].Nat Immunol,2007,8(6):578-583.
- [25] LIU K,VICTORA G D,SCHWICKERT T A,et al.In vivo analysis of dendritic cell development and homeostasis[J].Science,2009,324(5925):392-397.
- [26] COOK S J,LEE Q,WONG A C,et al.Differential chemokine receptor expression and usage by pre-cDC1 and pre-cDC2[J].Immunol Cell Biol,2018,96(10):1131-1139.
- [27] GRANOT T,SENDA T,CARPENTER D J,et al.Dendritic cells display subset and tissue-specific maturation dynamics over human life[J].Immunity,2017,46(3):504-515.
- [28] GEISSMANN F,JUNG S,LITTMAN D R.Blood monocytes consist of two principal subsets with distinct migratory properties[J].Immunity,2003,19(1):71-82.
- [29] SUNDERKÖTTER C,NIKOLIC T,DILLON M J,et al.Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response[J].J Immunol,2004,172(7):4410-4417.
- [30] BRISEÑO C G,HALDAR M,KRETZER N M,et al.Distinct transcriptional programs control cross-priming in classical and monocyte-derived dendritic cells[J].Cell Rep,2016,15(11):2462-2474.
- [31] HELFT J,BÖTTCHER J,CHAKRAVARTY P,et al.GM-CSF mouse bone marrow cultures comprise a hetero-geneous population of CD11c+MHCⅡ+ macrophages and dendritic cells[J].Immunity,2015,42(6):1197-1211.
- [32] SERBINA N V,SALAZAR-MATHER T P,BIRON C A,et al.TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection[J].Immunity,2003,19(1):59-70.
- [33] PLANTINGA M,GUILLIAMS M,VANHEERSWYNGHELS M,et al.Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen[J].Immunity,2013,38(2):322-335.
- [34] TAMOUTOUNOUR S,GUILLIAMS M,MONTA-NANA SANCHIS F,et al.Origins and functional specialization of macrophages and of conventional and mo-nocyte-derived dendritic cells in mouse skin[J].Immunity,2013,39(5):925-938.
- [35] SWIECKI M,COLONNA M.The multifaceted biology of plasmacytoid dendritic cells[J].Nat Rev Immunol,2015,15(8):471-485.
- [36] DRESS R J,WONG A Y,GINHOUX F.Homeostatic control of dendritic cell numbers and differentiation[J].Immunol Cell Biol,2018,96(5):463-476.
- [37] ALCULUMBRE S G,SAINT-ANDRÉ V,DI DOMIZIO J,et al.Diversification of human plasmacytoid predendritic cells in response to a single stimulus[J].Nat Immunol,2018,19(1):63-75.
- [38] NAGASAWA M,SCHMIDLIN H,HAZEKAMP M G,et al.Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B[J].Eur J Immunol,2008,38(9):2389-2400.
- [39] RODRIGUES P F,ALBERTI-SERVERA L,EREMIN A,et al.Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells[J].Nat Immunol,2018,19(7):711-722.
- [40] DRESS R J,DUTERTRE C A,GILADI A,et al.Plasmacytoid dendritic cells develop from Ly6D+ lymphoid progenitors distinct from the myeloid lineage[J].Nat Immunol,2019,20(7):852-864.
- [41] DOEBEL T,VOISIN B,NAGAO K.Langerhans cells:the macrophage in dendritic cell clothing[J].Trends Immunol,2017,38(11):817-828.
- [42] SCHULZ C,GOMEZ PERDIGUERO E,CHORRO L,et al.A lineage of myeloid cells independent of Myb and hematopoietic stem cells[J].Science,2012,336(6077):86-90.
- [43] KUBO A,NAGAO K,YOKOUCHI M,et al.External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers[J].J Exp Med,2009,206(13):2937-2946.
- [44] ENGLEMAN E G,BENIKE C J,GRUMET F C,et al.Activation of human T lymphocyte subsets:helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens[J].J Immunol,1981,127(5):2124-2129.
- [45] DOYLE C,STROMINGER J L.Interaction between CD4 and class Ⅱ MHC molecules mediates cell adhesion[J].Nature,1987,330(6145):256-259.
- [46] REIS E SOUSA C,STAHL P D,AUSTYN J M.Phagocytosis of antigens by Langerhans cells in vitro[J].J Exp Med,1993,178(2):509-519.
- [47] HOFFMANN E,KOTSIAS F,VISENTIN G,et al.Autonomous phagosomal degradation and antigen presentation in dendritic cells[J].Proc Natl Acad Sci USA,2012,109(36):14556-14561.
- [48] GARRETT W S,CHEN L M,KROSCHEWSKI R,et al.Developmental control of endocytosis in dendritic cells by Cdc42[J].Cell,2000,102(3):325-334.
- [49] BONIFAZ L C,BONNYAY D P,CHARALAMBOUS A,et al.In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination[J].J Exp Med,2004,199(6):815-824.
- [50] SALLUSTO F,CELLA M,DANIELI C,et al.Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class Ⅱ compartment:downregulation by cytokines and bacterial products[J].J Exp Med,1995,182(2):389-400.
- [51] NORBURY C C,CHAMBERS B J,PRESCOTT A R,et al.Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class Ⅰ presentation of exogenous soluble antigen by bone marrow-derived dendritic cells[J].Eur J Immunol,1997,27(1):280-288.
- [52] OUCHI T,KUBO A,YOKOUCHI M,et al.Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome[J].J Exp Med,2011,208(13):2607-2613.
- [53] NIESS J H,BRAND S,GU X B,et al.CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance[J].Science,2005,307(5707):254-258.
- [54] RESCIGNO M,URBANO M,VALZASINA B,et al.Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria[J].Nat Immunol,2001,2(4):361-367.
- [55] OUWEHAND K,SANTEGOETS S J A M,BRUYNZEEL D P,et al.CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis[J].Eur J Immunol,2008,38(11):3050-3059.
- [56] KISSENPFENNIG A,HENRI S,DUBOIS B,et al.Dynamics and function of Langerhans cells in vivo:dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells[J].Immunity,2005,22(5):643-654.
- [57] OCHIAI S,ROEDIGER B,ABTIN A,et al.CD326loCD103loCD11blo dermal dendritic cells are activated by thymic stromal lymphopoietin during contact sensitization in mice[J].J Immunol,2014,193(5):2504-2511.
- [58] WORBS T,BODE U,YAN S,et al.Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells[J].J Exp Med,2006,203(3):519-527.
- [59] KNOOP K A,MILLER M J,NEWBERRY R D.Transepithelial antigen delivery in the small intestine:different paths,different outcomes[J].Curr Opin Gastroenterol,2013,29(2):112-118.
- [60] JANG M H,KWEON M N,IWATANI K,et al.Intestinal villous M cells:an antigen entry site in the mucosal epithelium[J].Proc Natl Acad Sci USA,2004,101(16):6110-6115.
- [61] TORDESILLAS L,LOZANO-OJALVO D,DUNKIN D,et al.PDL2+ CD11b+ dermal dendritic cells capture topical antigen through hair follicles to prime LAP+ Tregs[J].Nat Commun,2018,9(1):5238.
- [62] DECKERS J,SICHIEN D,PLANTINGA M,et al.Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells[J].J Allergy Clin Immunol,2017,140(5):1364-1377.
- [63] BOLLAMPALLI V P,HARUMI YAMASHIRO L,FENG X G,et al.BCG skin infection triggers IL-1R-MyD88-dependent migration of EpCAMlow CD11bhigh skin dendritic cells to draining lymph node during CD4+ T-cell priming[J].PLoS Pathog,2015,11(10):e1005206.
- [64] CONNOR L M,TANG S C,CAMBERIS M,et al.Helminth-conditioned dendritic cells prime CD4+ T cells to IL-4 production in vivo[J].J Immunol,2014,193(6):2709-2717.
- [65] GERNER M Y,TORABI-PARIZI P,GERMAIN R N.Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens[J].Immunity,2015,42(1):172-185.
- [66] GERNER M Y,CASEY K A,KASTENMULLER W,et al.Dendritic cell and antigen dispersal landscapes regulate T cell immunity[J].J Exp Med,2017,214(10):3105-3122.
- [67] GODT H,LEHMANN J.Enzyme activities in tooth structure after treatment of an enamel-dentin fracture[J].Zahnarztl Prax,1972,23(5):122.
- [68] DUDZIAK D,KAMPHORST A O,HEIDKAMP G F,et al.Differential antigen processing by dendritic cell subsets in vivo[J].Science,2007,315(5808):107-111.
- [69] LEHMANN C H K,BARANSKA A,HEIDKAMP G F,et al.DC subset-specific induction of T cell responses upon antigen uptake via Fcγ receptors in vivo[J].J Exp Med,2017,214(5):1509-1528.
- [70] CAMINSCHI I,PROIETTO A I,AHMET F,et al.The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement[J].Blood,2008,112(8):3264-3273.
- [71] VANDER LUGT B,KHAN A A,HACKNEY J A,et al.Transcriptional programming of dendritic cells for enhanced MHC class Ⅱ antigen presentation[J].Nat Immunol,2014,15(2):161-167.
- [72] VAN PANHUYS N.TCR signal strength alters T-DC activation and interaction times and directs the outcome of differentiation[J].Front Immunol,2016,7:6.
- [73] HILLIGAN K L,RONCHESE F.Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses[J].Cell Mol Immunol,2020,17(6):587-599.
- [74] LUDA K M,JOERIS T,PERSSON E K,et al.IRF8 transcription-factor-dependent classical dendritic cells are essential for intestinal T cell homeostasis[J].Immunity,2016,44(4):860-874.
- [75] CHUDNOVSKIY A,MORTHA A,KANA V,et al.Host-protozoan interactions protect from mucosal infections through activation of the inflammasome[J].Cell,2016,167(2):444-456.
- [76] AFKARIAN M,SEDY J R,YANG J,et al.T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells[J].Nat Immunol,2002,3(6):549-557.
- [77] RUFFELL B,CHANG-STRACHAN D,CHAN V,et al.Macrophage IL-10 blocks CD8+ T cell-dependent res-ponses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells[J].Cancer Cell,2014,26(5):623-637.
- [78] GROOM J R,RICHMOND J,MUROOKA T T,et al.CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation[J].Immunity,2012,37(6):1091-1103.
- [79] JANKOVIC D,KULLBERG M C,HIENY S,et al.In the absence of IL-12,CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-)setting[J].Immunity,2002,16(3):429-439.
- [80] CONNOR L M,TANG S C,COGNARD E,et al.Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles[J].J Exp Med,2017,214(1):125-142.
- [81] JANSS T,MESNIL C,PIROTTIN D,et al.Interferon response factor-3 promotes the pro-Th2 activity of mouse lung CD11b+ conventional dendritic cells in res-ponse to house dust mite allergens[J].Eur J Immunol,2016,46(11):2614-2628.
- [82] KORN T,BETTELLI E,OUKKA M,et al.IL-17 and Th17 cells[J].Annu Rev Immunol,2009,27:485-517.
- [83] GHORESCHI K,LAURENCE A,YANG X P,et al.Generation of pathogenic TH17 cells in the absence of TGF-β signalling[J].Nature,2010,467(7318):967-971.
- [84] CHUNG Y,CHANG S H,MARTINEZ G J,et al.Critical regulation of early Th17 cell differentiation by interleukin-1 signaling[J].Immunity,2009,30(4):576-587.
- [85] JOSEFOWICZ S Z,LU L F,RUDENSKY A Y.Regulatory T cells:mechanisms of differentiation and function[J].Annu Rev Immunol,2012,30:531-564.
- [86] SCHIRMER C,KLEIN C,VON BERGEN M,et al.Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells[J].Blood,2010,116(10):1715-1725.
- [87] RIOL-BLANCO L,ORDOVAS-MONTANES J,PERRO M,et al.Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation[J].Nature,2014,510(7503):157-161.
- [88] GUILLIAMS M,CROZAT K,HENRI S,et al.Skin-draining lymph nodes contain dermis-derived CD103- dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells[J].Blood,2010,115(10):1958-1968.
- [89] SUN C M,HALL J A,BLANK R B,et al.Small intestine lamina propria dendritic cells promote de novo ge-neration of Foxp3 Treg cells via retinoic acid[J].J Exp Med,2007,204(8):1775-1785.
- [90] CHOI Y S,KAGEYAMA R,ETO D,et al.ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6[J].Immunity,2011,34(6):932-946.
- [91] NURIEVA R I,CHUNG Y,MARTINEZ G J,et al.Bcl6 mediates the development of T follicular helper cells[J].Science,2009,325(5943):1001-1005.
- [92] JOHNSTON R J,POHOLEK A C,DITORO D,et al.Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation[J].Science,2009,325(5943):1006-1010.
- [93] BALLESTEROS-TATO A,RANDALL T D,LUND F E,et al.T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite[J].Immunity,2016,44(2):259-273.
- [94] CEROVIC V,HOUSTON S A,WESTLUND J,et al.Lymph-borne CD8α+ dendritic cells are uniquely able to cross-prime CD8+T cells with antigen acquired from intestinal epithelial cells[J].Mucosal Immunology,2015,8(1):38-48.
- [95] COOMBES J L,SIDDIQUI K R R,ARANCIBIA-CRCAMO C V,et al.A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism[J].J Exp Med,2007,204(8):1757-1764.
- [96] JAENSSON-GYLLENBÄCK E,KOTARSKY K,ZAPATA F,et al.Bile retinoids imprint intestinal CD103+ dendri-tic cells with the ability to generate gut-tropic T cells[J].Mucosal Immunol,2011,4(4):438-447.
- [97] JAENSSON E,URONEN-HANSSON H,PABST O,et al.Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans[J].J Exp Med,2008,205(9):2139-2149.
- [98] ESTERHÁZY D,LOSCHKO J,LONDON M,et al.Classical dendritic cells are required for dietary antigen-mediated induction of peripheral Treg cells and tolerance[J].Nat Immunol,2016,17(5):545-555.
- [99] ESTERHÁZY D,CANESSO M C C,MESIN L,et al.Compartmentalized gut lymph node drainage dictates adaptive immune responses[J].Nature,2019,569(7754):126-130.
- [100] VEENBERGEN S,VAN BERKEL L A,DU PRÉ M F,et al.Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103+ dendritic cells[J].Mucosal Immunol,2016,9(4):894-906.
- [101] LUCIANI C,HAGER F T,CEROVIC V,et al.Dendritic cell functions in the inductive and effector sites of intestinal immunity[J].Mucosal Immunol,2022,15(1):40-50.
- [102] BAIN C C,MONTGOMERY J,SCOTT C L,et al.TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine[J].Nat Commun,2017,8(1):620.
- [103] WORTHINGTON J J,CZAJKOWSKA B I,MELTON A C,et al.Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8[J].Gastroenterology,2011,141(5):1802-1812.
- [104] CHNG S H,KUNDU P,DOMINGUEZ-BRAUER C,et al.Ablating the aryl hydrocarbon receptor(AhR)in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity[J].Sci Rep,2016,6:23820.
- [105] NEWBERRY R D,MCDONOUGH J S,STENSON W F,et al.Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria:directing the tone of the intestinal immune response[J].J Immunol,2001,166(7):4465-4472.
- [106] DELGADO M,GONZALEZ-REY E,GANEA D.The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells[J].J Immunol,2005,175(11):7311-7324.
- [107] SINGH N,GURAV A,SIVAPRAKASAM S,et al.Activation of Gpr109a,receptor for niacin and the commensal metabolite butyrate,suppresses colonic inflammation and carcinogenesis[J].Immunity,2014,40(1):128-139.
- [108] CORDING S,WAHL B,KULKARNI D,et al.The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes[J].Mucosal Immunol,2014,7(2):359-368.
- [109] HAMMERSCHMIDT S I,AHRENDT M,BODE U,et al.Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo[J].J Exp Med,2008,205(11):2483-2490.
- [110] STEINMAN R M.Decisions about dendritic cells:past,present,and future[J].Annu Rev Immunol,2012,30(1):1-22.
- [111] CHIANG M C,TULLETT K M,LEE Y S,et al.Differential uptake and cross-presentation of soluble and necrotic cell antigen by human DC subsets[J].Eur J Immunol,2016,46(2):329-339.
- [112] BINNEWIES M,MUJAL A M,POLLACK J L,et al.Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity[J].Cell,2019,177(3):556-571.
- [113] SITTIG S P,BAKDASH G,WEIDEN J,et al.A comparative study of the T cell stimulatory and polarizing capacity of human primary blood dendritic cell subsets[J].Mediators Inflamm,2016,2016:3605643.
- [114] SEGURA E,DURAND M,AMIGORENA S.Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells[J].J Exp Med,2013,210(5):1035-1047.
- [115] WILLIAMS J W,TJOTA M Y,CLAY B S,et al.Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation[J].Nat Commun,2013,4:2990.
- [116] ROWSHANRAVAN B,HALLIDAY N,SANSOM D M.CTLA-4:a moving target in immunotherapy[J].Blood,2018,131(1):58-67.
- [117] SAOULLI K,LEE S Y,CANNONS J L,et al.CD28-independent,TRAF2-dependent costimulation of res-ting T cells by 4-1BB ligand[J].J Exp Med,1998,187(11):1849-1862.
- [118] DANNULL J,NAIR S,SU Z,et al.Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand[J].Blood,2005,105(8):3206-3213.
- [119] GREWAL I S,FLAVELL R A.The role of CD40 li-gand in costimulation and T-cell activation[J].Immunol Rev,1996,153:85-106.
- [120] BUCHAN S L,FALLATAH M,THIRDBOROUGH S M,et al.PD-1 blockade and CD27 stimulation activate distinct transcriptional programs that synergize for CD8+ T-cell-driven antitumor immunity[J].Clin Can-cer Res,2018,24(10):2383-2394.
- [121] WANG S,ZHU G,CHAPOVAL A I,et al.Costimulation of T cells by B7-H2,a B7-like molecule that binds ICOS[J].Blood,2000,96(8):2808-2813.
- [122] REIZIS B,BUNIN A N,GHOSH H S,et al.Plasmacytoid dendritic cells:recent progress and open questions[J].Annu Rev Immunol,2011,29:163-183.
- [123] HASHIMOTO H,UEDA R,NARUMI K,et al.Type Ⅰ IFN gene delivery suppresses regulatory T cells within tumors[J].Cancer Gene Therapy,2014,21(12):532-541.
- [124] VAISHAMPAYAN U,ABRAMS J,DARRAH D,et al.Active immunotherapy of metastatic melanoma with allogeneic melanoma lysates and interferon α[J].Clin Cancer Res,2002,8(12):3696-3701.
- [125] JIANG W,ZHANG C,TIAN Z,et al.hIFN-α gene modification augments human natural killer cell line anti-human hepatocellular carcinoma function[J].Gene Therapy,2013,20(11):1062-1069.
- [126] ZWIRNER N W,ZIBLAT A.Regulation of NK cell activation and effector functions by the IL-12 family of cytokines:the case of IL-27[J].Front Immunol,2017,8:25.
- [127] ZHU J M,LIU J Q,SHI M,et al.IL-27 gene therapy induces depletion of Tregs and enhances the efficacy of cancer immunotherapy[J].JCI Insight,2018,3(7):e98745.
- [128] SPRANGER S,DAI D,HORTON B,et al.Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy[J].Cancer Cell,2017,31(5):711-723.
- [129] SPRANGER S,BAO R Y,GAJEWSKI T F.Melanoma intrinsic β-catenin signalling prevents anti-tumour immunity[J].Nature,2015,523(7559):231-235.
- [130] GOTTFRIED E,KUNZ-SCHUGHART L A,EBNER S,et al.Tumor-derived lactic acid modulates dendritic cell activation and antigen expression[J].Blood,2006,107(5):2013-2021.
- [131] ZONG J B,KESKINOV A A,SHURIN G V,et al.Tumor-derived factors modulating dendritic cell function[J].Cancer Immunol Immunother,2016,65(7):821-833.
- [132] TRAN C W,GOLD M J,GARCIA-BATRES C,et al.Hypoxia-inducible factor 1 α limits dendritic cell stimulation of CD8 T cell immunity[J].PLoS One,2020,15(12):e0244366.
- [133] SILVA-VILCHES C,RING S,MAHNKE K.ATP and its metabolite adenosine as regulators of dendritic cell activity[J].Front Immunol,2018,9:2581.
- [134] APETOH L,GHIRINGHELLI F,TESNIERE A,et al.Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy[J].Nat Med,2007,13(9):1050-1059.
- [135] HU Z L,TENG X L,ZHANG T Y,et al.SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function[J].Mol Cell,2021,81(5):940-952.
- [136] SANTOS P M,MENK A V,SHI J,et al.Tumor-derived α-fetoprotein suppresses fatty acid metabolism and oxidative phosphorylation in dendritic cells[J].Cancer Immunol Res,2019,7(6):1001-1012.
- [137] CUBILLOS-RUIZ J R,SILBERMAN P C,RUTKOWSKI M R,et al.ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeo-stasis[J].Cell,2015,161(7):1527-1538.
- [138] HERBER D L,CAO W,NEFEDOVA Y,et al.Lipid accumulation and dendritic cell dysfunction in cancer[J].Nat Med,2010,16(8):880-886.
- [139] MERAD M,SATHE P,HELFT J,et al.The dendritic cell lineage:ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting[J].Annu Rev Immunol,2013,31(1):563-604.
- [140] MUNN D H,MELLOR A L.IDO in the tumor microenvironment:inflammation,counter-regulation,and tole-rance[J].Trends in Immunology,2016,37(3):193-207.
- [141] GABRILOVICH D,ISHIDA T,OYAMA T,et al.Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo[J].Blood,1998,92(11):4150-4166.
- [142] KIM K D,LIM H Y,LEE H G,et al.Apolipoprotein A-I induces IL-10 and PGE2 production in human mono-cytes and inhibits dendritic cell differentiation and maturation[J].Biochem Biophys Res Commun,2005,338(2):1126-1136.
- [143] TERRA M,OBERKAMPF M,FAYOLLE C,et al.Tumor-derived TGFβ alters the ability of plasmacytoid dendritic cells to respond to innate immune signaling[J].Cancer Res,2018,78(11):3014-3026.
- [144] COMBES A,CAMOSSETO V,N'GUESSAN P,et al.BAD-LAMP controls TLR9 trafficking and signalling in human plasmacytoid dendritic cells[J].Nat Commun,2017,8(1):913.
- [145] OBEID M,TESNIERE A,GHIRINGHELLI F,et al.Calreticulin exposure dictates the immunogenicity of cancer cell death[J].Nat Med,2007,13(1):54-61.
- [146] CASARES N,PEQUIGNOT M O,TESNIERE A,et al.Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death[J].J Exp Med,2005,202(12):1691-1701.
- [147] MA Y T,ADJEMIAN S,MATTAROLLO S R,et al.Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells[J].Immunity,2013,38(4):729-741.
- [148] GALLUZZI L,BUQUÉ A,KEPP O,et al.Immunoge-nic cell death in cancer and infectious disease[J].Nat Rev Immunol,2017,17(2):97-111.
- [149] GHIRINGHELLI F,APETOH L,TESNIERE A,et al.Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors[J].Nat Med,2009,15(10):1170-1178.
- [150] DENG L F,LIANG H,XU M,et al.STING-dependent cytosolic DNA sensing promotes radiation-induced type Ⅰ interferon-dependent antitumor immunity in immunogenic tumors[J].Immunity,2014,41(5):843-852.
- [151] SÁNCHEZ-PAULETE A R,CUETO F J,MARTÍNEZ-LPEZ M,et al.Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells[J].Cancer Discov,2016,6(1):71-79.
- [152] ALLOATTI A,ROOKHUIZEN D C,JOANNAS L,et al.Critical role for Sec22b-dependent antigen cross-presentation in antitumor immunity[J].J Exp Med,2017,214(8):2231-2241.
- [153] CAUWELS A,VAN LINT S,PAUL F,et al.Delivering type Ⅰ interferon to dendritic cells empowers tumor eradication and immune combination treatments[J].Cancer Res,2018,78(2):463-474.
- [154] RAPP M,GRASSMANN S,CHALOUPKA M,et al.C-C chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells,tumor infiltration and therapeutic efficacy of adoptive T cell transfer[J].OncoImmunology,2016,5(3):e1105428.
- [155] 张曼,杨涛,石洋,等.DCs-CIK细胞免疫治疗联合化疗对晚期非小细胞肺癌疗效及安全性的影响[J].肿瘤,2014,34(4):361-365.
- [156] NEFEDOVA Y,HUANG M,KUSMARTSEV S,et al.Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer[J].J Immunol,2004,172(1):464-474.
- [157] NEFEDOVA Y,CHENG P Y,GILKES D,et al.Activation of dendritic cells via inhibition of Jak2/STAT3 signaling[J].J Immunol,2005,175(7):4338-4346.
- [158] 黄浩.腺相关病毒负载癌胚抗原制备DC疫苗的实验研究[D].广州:南方医科大学,2007.
- [159] MAZZOLINI G,ALFARO C,SANGRO B,et al.Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas[J].J Clin Oncol,2005,23(5):999-1010.
- [160] KANTOFF P W,HIGANO C S,SHORE N D,et al.Sipuleucel-T immunotherapy for castration-resistant prostate cancer[J].The New England Journal of Medicine,2010,363(5):411-422.
- [161] BARTH R J,Jr,FISHER D A,WALLACE P K,et al.A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients:tumor-specific immune responses are associated with improved survival[J].Clin Cancer Res,2010,16(22):5548-5556.
- [162] 张文颖,袁海花,姜斌,等.TRAIL基因修饰的树突状细胞诱导黑色素瘤细胞凋亡的实验研究[J].现代免疫学,2017,37(5):390-394.
- [163] 杨岩丽,王丹红,廖丽,等.DC疫苗联合细胞因子诱导的杀伤细胞治疗转移性肾癌的长期疗效分析[J].中国肿瘤生物治疗杂志,2019,26(6):695-699.
- [164] FAIENA I.A phase Ⅰ,open-label,dose-escalation,and cohort expansion study to evaluate the safety and immune response to autologous dendritic cells transduced with AdGMCA9(DC-AdGMCAIX)in patients with metastatic renal cell carcinoma[J].Journal of Immunotherapy,2020,43(9):273-282.
![表1 已有研究确定的小鼠DC亚群的特征标志基因和蛋白质[5-7]<br/>Tab.1 Characteristic gene and protein markers of described murine DC subsets as determined from published resources[5-7]](2022年03期/pic24.jpg)