(1.厦门大学生命科学学院,细胞应激生物学国家重点实验室,福建 厦门 361102; 2.厦门大学公共卫生学院,分子疫苗学和分子诊断学国家重点实验室,福建 厦门 361102)
(1.State Key Laboratory of Cellular Stress Biology,School of Life Sciences,Xiamen University,Xiamen 361102,China; 2.State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics,School of Public Health,Xiamen University,Xiamen 361102,China)
DOI: 10.6043/j.issn.0438-0479.202002013
备注
引言
肾脏是机体应对环境暴露的重要排泄、代谢解毒器官,肾脏免疫(包括先天免疫和适应性免疫)具有参与抵抗内、外源抗原侵害肾脏的功能.肾脏中驻留的先天免疫细胞既可直接吞噬“非自身”抗原,也可基于抗原呈递作用或释放细胞因子和炎性介质间接调控适应性免疫,但免疫系统过度激活则可能造成器官损伤和病变[1-2].外源化学物既可引起肾脏先天免疫应答,也可引起适应性免疫应答,其主要通过诱导肾脏内免疫细胞活化参与肾脏损伤[3].辅助性T细胞(Th细胞)可介导免疫级联反应,释放炎症因子,促进自身抗体生成,达到增强或减弱组织炎症的作用,参与肾脏适应性免疫[4].
本文综述了肾脏适应性免疫中不同类型Th细胞的功能,重点论述Th细胞在调控重金属、有机污染物、大气细颗粒物(PM2.5)、纳米材料等外源化学物诱导肾脏免疫反应中的作用和机制,以进一步了解适应性免疫在外源化学物诱导的肾脏毒性损伤中的作用,为其干预和治疗提供理论依据.
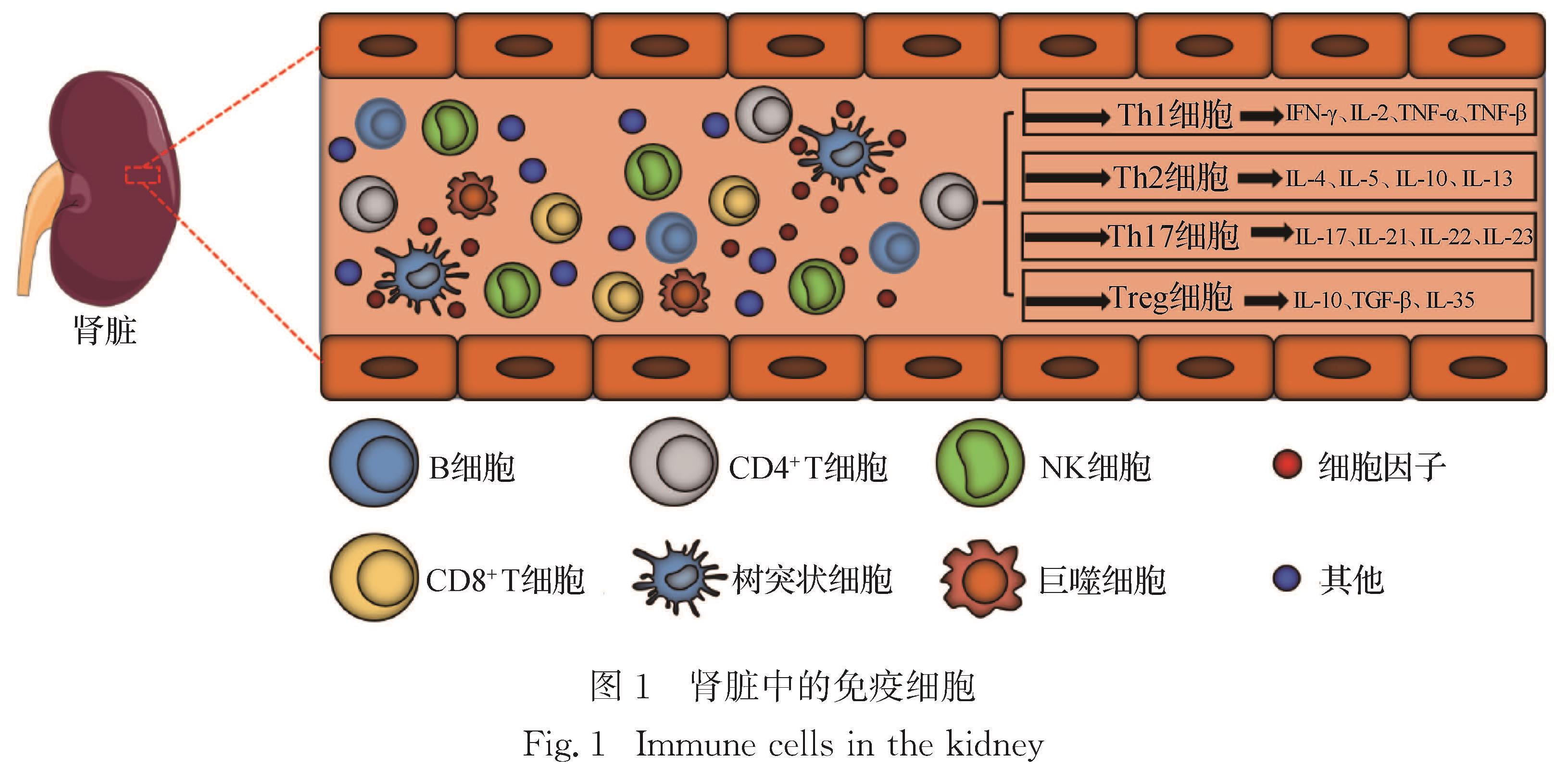
1 肾脏中T细胞的分类及功能
成人肾脏具有丰富的细胞亚群,包括内皮细胞、免疫细胞、成纤维细胞、成肌纤维细胞和上皮细胞亚群.如图1所示,其中免疫细胞亚群包括树突状细胞、巨噬细胞、自然杀伤细胞(NK细胞)、B细胞和T细胞[5].T细胞按表面分化簇抗原可分为CD4+ T细胞和CD8+ T细胞:其中,CD4+ T细胞主要分化为Th细胞和调节性T细胞(Treg细胞),可辅助调节B细胞和巨噬细胞活化; CD8+ T细胞主要分化为细胞毒性T淋巴细胞(CTL细胞),能特异性杀伤靶细胞,如
肿瘤细胞.Th细胞有多种亚型,包括Th1细胞、Th2细胞、Th9细胞、Th17细胞、Th22细胞和滤泡辅助性T细胞(Tfh细胞)等[6].Th1、Th2、Th17及Treg细胞是肾脏中主要的CD4+ T细胞亚群[2,6],研究这些CD4+ T细胞的数量、比例及其对肾脏免疫微环境的影响,是了解外源化学物诱导免疫性肾脏损伤发生机制的关键.1.1 Th1细胞Th1细胞是受细胞特异性转录因子T-bet调节的一类T细胞亚群,主要分泌干扰素(IFN)-γ、白细胞介素(IL)-2、肿瘤坏死因子(TNF)-α和TNF-β(图1),并可刺激实质细胞分泌IL-2、IL-6等促炎性细胞因子[7].Th1细胞主要参与细胞免疫反应、迟发性过敏(DTH)反应、CTL细胞成熟和NK细胞激活等[8-9].
Th1细胞在肾脏损伤和病变中发挥重要作用.一方面,Th1细胞在肾脏损伤和疾病进展早期发挥促进作用.例如:肾小球性肾炎是一种自身免疫性疾病,其进程中伴有T细胞聚集.Hopfer等[10]在肾小球性肾炎小鼠模型中检测到CD4+ T细胞在肾脏损伤后期积累,趋化因子Cxcl9、Cxcl10、Ccl3、Ccl4和Ccl5基因表达水平以及相应的趋化因子受体Cxcr3和Ccr5基因表达水平升高,提示在肾小球性肾炎中Th1细胞激活并介导肾脏炎症反应; Th1细胞缺失小鼠肾小管间质损伤和半月体肾小球肾炎形成显著减少,表明Th1细胞可能会促进肾小球肾炎进展[11-12].此外,糖尿病肾病患者血清中Th1细胞分泌的IFN-γ和TNF-α水平明显升高,而在诱导性多能干细胞注射治疗后IL-2水平下降,提示糖尿病肾病患者的Th1细胞分化异常[13-14].另一方面,Th1细胞可能在肾脏慢性损伤和疾病晚期发挥重要作用.例如,肾纤维化是大多数慢性肾脏疾病发展的结局之一,主要病理表现为肾脏间质成纤维细胞大量增殖激活和细胞外基质过度累积.Wen等[15]研究表明纤维化与Th1细胞介导的炎症反应密切相关; 缺乏Th1细胞分化能力的T-bet基因敲除小鼠单侧输尿管阻塞后,肾脏胶原蛋白沉积减少,纤维化程度降低,表明抑制肾脏Th1细胞分化可减轻肾脏慢性损伤中的纤维化进程.
以上研究表明:Th1细胞在肾小球肾炎等早期肾脏疾病中可能发挥促进作用; 而在肾脏纤维化等慢性损伤过程中,可能通过招募外周血巨噬细胞或其他免疫细胞控制炎症,减轻组织损伤.
1.2 Th2 细胞Th2细胞是以分泌IL-4、IL-5、IL-10和IL-13等细胞因子为主的一类CD4+ T细胞亚群(图1),它受信号传导及转录激活蛋白6(STAT6)和转录因子GATA结合蛋白3(GATA3)的调控[16].Th2细胞可抑制CD4+ T细胞向Th1细胞分化; Th2细胞还可释放炎症因子来诱导B细胞产生免疫球蛋白,刺激嗜碱性粒细胞或肥大细胞释放炎症因子,与体液免疫和过敏反应调节有关[17-18].
Th2细胞在多种肾脏免疫性疾病病变过程中也发挥关键作用.狼疮性肾炎是一种与系统性红斑狼疮有关的自身免疫性疾病,由免疫复合物在肾脏中的沉积引起.Selvaraja等[19]发现系统性红斑狼疮患者的血浆中IL-9和IL-10表达增加,且与疾病严重程度呈正相关,表明Th2细胞可能与系统性红斑狼疮发病有关.Stangou等[20]发现免疫球蛋白A(IgA)肾病患者中多数出现肾小球膜和毛细血管增生、肾小球硬化和肾小管间质纤维化,尿液中IL-4、IL-5和IL-13等Th2细胞相关炎症因子水平均升高.Deng等[21]在IgA肾病模型大鼠外周血中也检测到Th2细胞数目增多及促炎性细胞因子IL-6分泌增加.此外,Liu等[22]发现在IgA肾病患者的肾脏中出现CD4+ T细胞浸润,通过抗CD4抗体耗竭小鼠的CD4+T细胞后,肾脏中Th2细胞比例升高,单侧输尿管阻塞诱导的肾纤维化被显著抑制; 通过尾静脉注射活化的Th2细胞可恢复CD4+T细胞缺失小鼠肾纤维化程度,表明Th2细胞比例升高可加剧肾脏纤维化进程.这些研究表明Th2细胞及其分泌的细胞因子在肾脏纤维化等后期损伤过程中发挥促进作用.
在健康情况下,人体Th1/Th2细胞处于动态平衡状态,当机体受到外来刺激或发生损伤时该平衡状态可能被打破,进而引起疾病[23].尽管已观察到Th1和Th2细胞的比例在肾小球肾炎或弥漫性增生性狼疮肾炎等肾脏疾病中升高或降低,但它们在人类肾脏免疫性疾病中作用尚不明确[24].因此,探讨影响肾脏受到外源化学物暴露后干扰Th1/Th2细胞平衡的机制,可能有助于干预和治疗肾脏损伤.
1.3 Th17细胞Th17细胞是以表达维甲酸相关孤核受体γt(RORγt),分泌IL-17、IL-21、IL-22和IL-23等细胞因子和表达趋化因子受体(CCR6)为主要特征的一类CD4+T细胞亚群(图1),通过诱导巨噬细胞分泌促炎性细胞因子或募集嗜中性粒细胞浸润,诱导肾脏免疫毒性损伤以及自身免疫疾病[4,25].
Mok等[26]发现活动性狼疮肾炎患者的血清中IL-17水平显著高于健康人群,Jakiela等[27]在狼疮性肾炎患者的外周血中鉴定出Th17细胞比例升高,Orejudo等[28]在高血压性肾动脉硬化患者的肾脏炎性细胞浸润区域也发现Th17细胞.此外,动物实验发现Th17细胞与肾脏损伤和病变有关.Gan等[29]注射抗髓过氧化物酶IgG抗体构建肾小球肾炎小鼠模型,发现在IL-17A敲除小鼠中未检测到肾小球炎性指标异常,肾脏白细胞浸润显著减少,表明在肾小球肾炎等相关疾病中Th17细胞可能介导肾脏损伤发生.Mehrotra等[30]发现高盐饮食依赖于Th17细胞活化从而加重急性肾损伤大鼠疾病进程转化.Chung等[31]利用IL-17刺激原代人肾近曲小管上皮细胞后,IL-6和IL-8分泌增多,肾脏损伤分子1(KIM-1)的mRNA水平呈剂量依赖性上升,细胞间相互作用相关基因CDH1表达下降,E-钙黏着蛋白表达减少,提示IL-17A可能影响肾脏结构和功能[32].综上可知Th17细胞介导的免疫反应在肾脏损伤和病变中发挥诱导作用.
1.4 Treg细胞Treg细胞分为胸腺来源和外周血来源,是一类表达叉头样转录因子Foxp3的CD4+CD25+T细胞亚群,通过细胞间接触以及分泌IL-10、IL-35和肿瘤生长因子β(TGF-β)等抑炎性细胞因子,发挥抑制肾脏炎症反应及调控组织修复的功能[33](图1).
Zhu等[34]研究发现慢性肾脏疾病患者的外周血中Treg细胞比例低于健康人群,并随着疾病加剧而降低.类似地,狼疮性肾炎患者的外周血中Treg细胞比例显著低于健康人群,尿液中Foxp3基因表达水平升高,提示Treg细胞功能随着肾炎加剧而受损[35-36].在小鼠肾脏缺血再灌注模型中,Treg细胞数目在损伤发生后增多,而耗竭或移植Treg细胞则可加剧或减轻肾小管损伤,并发现Treg细胞对肾脏损伤的修复过程可能是通过抑制其他T细胞亚群的炎症因子分泌实现的[37-38].Yao等[39]利用荜茇酰胺抑制脾脏中Th17细胞和增加Treg细胞,可减轻小鼠狼疮性肾炎中的免疫复合物沉积并改善肾损伤.综上所述,Treg细胞在肾脏疾病的发展中起着重要作用,提高Treg细胞比例或移植Treg细胞的策略已被证明在预防或治疗肾脏疾病中具有很大的应用潜力[40-42].
在健康情况下,肾脏Th17细胞发挥促炎作用,Treg细胞发挥抑炎作用,两者功能上互相抑制[43].当Th17/Treg细胞平衡状态被打破时,人体免疫环境发生紊乱,包括Th17/Treg细胞功能和数目改变、炎症因子分泌失调及巨噬细胞激活等,导致免疫系统失去正常的调节功能[44-45].因此,需要进一步研究外源化学物影响肾脏Th17/Treg细胞稳态的机制,为维持肾脏正常功能和控制肾脏疾病发生提供科学依据.
2 Th细胞在外源化学物诱导肾脏损伤中的作用
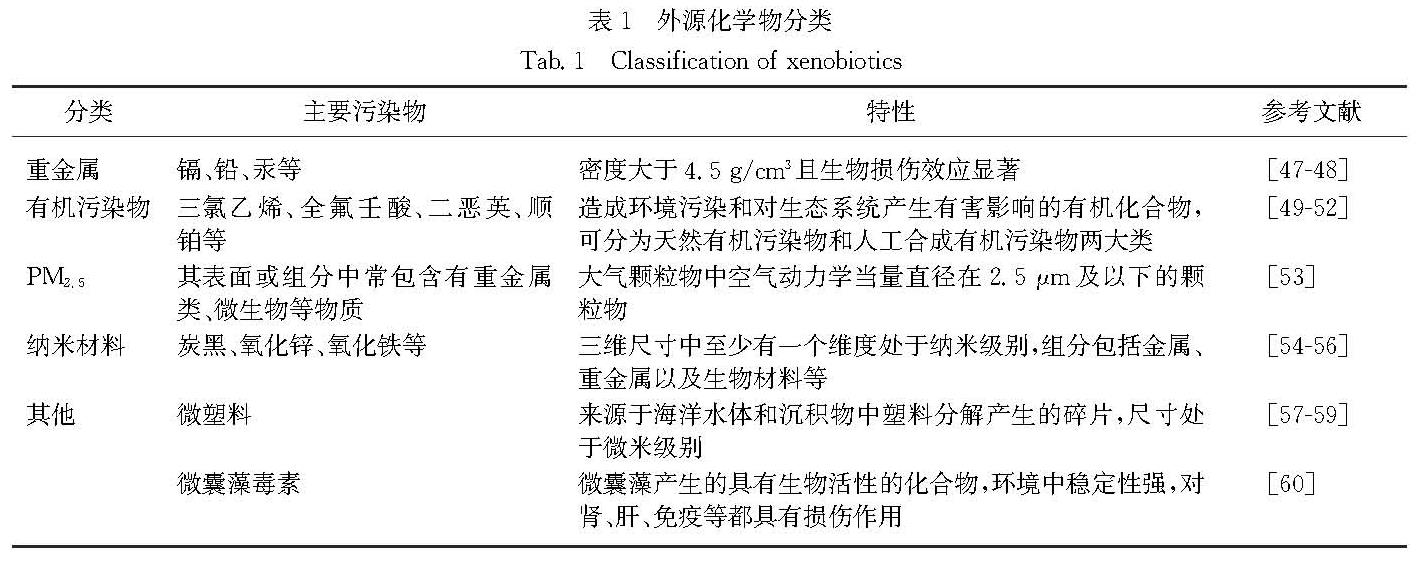
外源化学物是指在人类生活的外界环境中存在、可能与机体接触并进入机体,且在体内呈现一定生物学作用的一些化学物质[46].常见外源化学物包括重金属、有机污染物、PM2.5、纳米材料、微塑料和微囊藻毒素(表1),通过食物、饮水、空气等多种途径进入机体,可对机体造成损伤作用,包括引起肾脏免疫毒性损伤[61-63].下文将分别论述Th细胞在这些外源化学物诱导的肾脏免疫毒性损伤中的作用.
2.1 重金属诱导的肾脏损伤环境污染和职业接触重金属是引起机体损伤的高危因素.汞、铅、镉、铬等重金属及类金属砷可以通过食物、水、空气等途径进入人体,经血液循环分布到包括肾脏在内的多个主要器官,引起肾毒性损伤[47-48].
Langworth等[64]发现暴露于汞蒸气的工人血汞、尿汞及血肌酐水平均高于未暴露人群,提示人群汞暴露与肾损伤的发生呈正相关.Kim等[65]将雄性BALB/c小鼠饮水暴露于37.5 μg/mL汞14 d,小鼠外周血中红细胞数目减少而白细胞数目增加,肾脏中Tnf-α、
Ifn-g、Il-12和主要组织相容性复合体Ⅱ(Mhc Ⅱ)等炎症因子基因表达水平升高,而脾脏中CD3+、CD4+和CD8+ T细胞数量减少.Pillet等[66]将去卵巢的雌性大鼠和假手术完整雌性大鼠饮水暴露于25 μg/mL 氯化镉28 d,发现去卵巢的雌性大鼠胸腺T细胞中CD4+CD8- T细胞的比例较假手术组大鼠增多,可能与低剂量镉结合并激活雌激素受体ERα,诱导雌激素依赖性基因表达有关,提示需进一步考虑镉的类雌激素效应和实验动物性别在镉引起的免疫毒性风险中的影响.砷暴露还可引起胸腺发育异常、T细胞亚群分化改变等[67-68].Gera等[69]将雄性BALB/c小鼠暴露于0.038,0.38和3.8 μg/mL亚砷酸钠,发现7,15和30 d后,肾脏组织更易受细菌感染,且胸腺和脾脏中CD4+T细胞比例显著增加,脾脏中Treg细胞数量也增多,表明砷暴露会干扰免疫器官中T细胞分化,从而影响机体免疫功能.
然而,目前鲜有研究关注肾脏T细胞分化情况以及Th细胞在金属诱导的肾脏损伤中的作用.因此,Th细胞在金属诱导肾脏损伤中的作用和机制研究是将来值得关注的一个重要方向.
2.2 有机污染物诱导的肾脏损伤有机污染物主要包括萜烯类、黄曲霉素等天然有机污染物,多环芳烃、多氯联苯、全氟化物等持久性污染物,以及农药、食品添加剂、药品等人工合成有机污染物[49].多种有机污染物可以导致肾脏损伤和免疫反应.例如:Wang等[50]发现雌性BALB/c小鼠经皮肤暴露于三氯乙烯20 d后,血清中尿素氮和肌酐水平升高,肾脏炎症细胞浸润,肾小管上皮细胞液泡变性.Coutinho等[70]发现雌性Wistar大鼠以100和500 ng/kg氯化三丁基锡灌胃处理15 d后,肾脏中出现锡积累,并伴随肾小管液泡变性、凋亡和炎性细胞浸润.Fang等[51]研究证实小鼠口服全氟壬酸14 d会导致体质量以及胸腺和脾脏质量降低,其中,1 mg/kg全氟壬酸处理组的小鼠脾脏中CD8+T细胞比例下降,5 mg/kg全氟壬酸处理组的小鼠脾脏中CD11c+、F4/80+和CD49b+细胞比例下降,同时脾脏中Th1和Th2细胞分泌的标志性细胞因子IFN-γ和IL-4减少.Mustafa等[52]研究发现C57BL/6孕鼠暴露于二恶英后,5 μg/kg暴露组的雄性子代脾脏中CD4-CD8+ T细胞比例降低,雌性子代淋巴结中CD4+CD8- T细胞比例降低,所有子代小鼠均显示肾脏中免疫球蛋白和肾小球补体C3沉积增加,提示二恶英暴露可能导致子代小鼠肾小球肾炎和自身免疫功能改变.多球壳菌素是一种真菌代谢产物,最初被用作抗生素和免疫抑制剂.雄性BALB/c小鼠连续以0.1,0.3和1.0 mg/kg多球壳菌素腹腔注射5 d后,胸腺中CD4+T细胞和CD4+CD8+ T细胞比例显著下降[71].以上研究表明有机污染物可导致动物免疫毒性,主要表现为胸腺和脾脏等免疫器官损伤、T细胞活化以及外周血中促炎因子水平升高,推测可能影响肾脏等供血量高的器官.
在顺铂诱导的小鼠急性肾损伤模型中,肾脏CD4+ T细胞比例升高,炎性Th1和Th17细胞数目增多,肾脏移植Treg细胞可提高顺铂处理小鼠存活率,降低促炎因子水平和缓解肾脏损伤,而耗竭内源性Treg细胞则加剧了顺铂诱导的小鼠肾脏损伤[52,72-73].由此可见,有机污染物可能通过诱导肾脏T细胞向Th1或Th17细胞分化、抑制其向Treg细胞分化,诱导促炎性细胞因子分泌,从而介导肾脏损伤发生.
2.3 PM2.5诱导的肾脏损伤PM2.5容易经呼吸进入循环系统并分布到身体各个器官[53].肾脏损伤与PM2.5暴露有关,但是否与免疫调控有关尚不清楚[74].Liu等[75]利用染色质免疫共沉淀技术对不同PM2.5暴露水平的人群血液样本进行分析,发现部分参与免疫功能的基因的组蛋白3赖氨酸27(H3K27)乙酰化修饰发生改变,提示PM2.5介导了人群炎症和免疫反应发生.Drela等[76]将雄性BALB/c小鼠按170 mg/kg单次腹腔注射空气悬浮颗粒物(直径0.3~10 μm)72 h后,小鼠的胸腺细胞数量减少,胸腺中参与调节突触形成的CD28- T细胞比例升高,CD28+ T细胞的比例下降,从而引起免疫系统紊乱.Chen等[77]研究发现C57BL/6孕鼠暴露于PM2.5后,脾脏中CD3+CD4+ T细胞增多,其雌性后代胸腺中CD4+CD25+ Treg细胞减少.Park等[78]发现ICR小鼠暴露于PM2.5后,血液中IL-12和IFN-γ等Th1细胞相关细胞因子分泌增多,CD4+T细胞与CD8+ T细胞的比例下降.Aztatzi-Aguilar等[79]发现雄性大鼠亚慢性PM2.5暴露后,早期肾脏功能指标肾小球滤过率水平降低,尿液早期肾脏损害标志物β2-微球蛋白增加,肾皮质中IL-6、IL-1β、TNF-α、IL-4、IL-10、IL-17A、人巨噬细胞炎性蛋白2(MIP-2)等炎症因子水平均显著下降,表明亚慢性PM2.5暴露可引起肾脏内免疫抑制、氧化应激以及肾脏生理功能改变.这些研究均提示PM2.5暴露可引起包括肾脏在内的多器官发生免疫反应,释放炎症因子造成器官损伤,但肾脏内T细胞活化在PM2.5引起肾脏损伤中的作用和机制仍不清楚,有待更深入研究.
2.4 纳米材料诱导的肾脏损伤纳米材料已广泛应用于生物医药、化妆品、食品等领域,其生物安全性也越来越受关注[3].纳米材料暴露可导致肾脏组织损伤和炎症反应.例如:Heidai-Moghadam等[54]报道50 mg/kg纳米氧化锌处理雄性大鼠14 d后,血浆尿酸、肌酐和尿素氮等肾脏损伤标志物的水平升高,且肾脏内肾近曲小管损伤,红细胞聚积,炎性细胞浸润和肾小球直径缩小,表明纳米氧化锌可引起肾脏免疫损伤.ICR雄性小鼠单次气管内滴注球形纳米氧化铁28 d,可破坏肺细胞因子平衡,并诱导Th2型炎症反应; 单次气管内滴注针状纳米氧化铁90 d,则因肺长期蓄积而引起Th1型炎症反应[56,80].Shimizu等[55]发现ICR孕鼠在妊娠第5天和第9天鼻内滴注炭黑纳米材料悬浮液后,子代脾脏中CD3+T细胞和CD4+T细胞减少.类似地,Chu等[81]发现长期暴露于炭黑纳米材料的雄性SD大鼠,其外周血中白细胞、单核细胞和中性粒细胞的数量增加,CD4+T细胞增多,CD4+T细胞与CD8+T细胞比例升高,提示吸入炭黑可诱导全身炎症和免疫反应.雌性BALB/c小鼠皮下注射炭黑则可诱导脾脏及骨髓中T细胞向Th1细胞分化[82].综上可知,诸多纳米材料可引起免疫系统异常,而肾脏作为纳米材料毒性的一个主要靶器官,目前尚无直接相关研究报道Th细胞在纳米材料肾毒性损伤中的作用.深入研究Th细胞在纳米材料诱导肾损伤中的作用和机制将为探索减轻纳米材料毒性提供一种新思路.
2.5 其他外源化学物诱导的肾脏损伤微塑料是指尺寸小于5 mm的塑料微颗粒,主要来源于塑料材料自身降解、化妆品及工业塑料生产,包括聚丙烯(PP)、聚乙烯(PE)、聚苯乙烯(PS)、聚氯乙烯(PVC)等[57-59].Deng等[83]研究发现ICR小鼠暴露于微塑料后,可在肾脏、肝脏和肠道中观察到微塑料聚积,肝脏组织经苏木精-伊红染色可见肝脏炎症和脂滴,提示微塑料可经循环系统转运至其他器官而引起炎症反应和脂质代谢紊乱.Limonta等[59]报道成年斑马鱼(Danio rerio)暴露于环境浓度的PS和PE混合物后,肠黏膜组织的完整性被破坏,白细胞数量增加,肝脏免疫和代谢途径相关基因异常表达.C57BL/6小鼠在摄入PE后,血清中细胞因子IL-1α、IL-6和IL-9的水平均升高,且脾脏中Treg细胞比例显著下降[84].以上研究提示微塑料这一新型环境污染物可以破坏机体免疫功能,血液中促炎性细胞因子水平升高,可能会造成肾脏等含血量高的器官损伤.
微囊藻毒素是一种具有生物活性和毒性的藻类代谢产物[63].Yi等[85]研究发现小鼠慢性暴露于高剂量微囊藻毒素可引起肾脏功能紊乱,包括肾脏滤过功能降低、肾小球肿胀、白细胞浸润增加等.Palikova等[86]研究发现Wistar大鼠喂食含有微囊藻毒素的饲料28 d后,暴露组大鼠外周血中CD4+CD8+T细胞比例均显著升高,脾脏中CD8+T细胞比例也显著升高.Dar等[87]研究发现BALB/c小鼠暴露于微囊藻毒素15 d后,血清中促炎性细胞因子IL-6、TNF-α和IL-17分泌增多,抑炎性细胞因子IFN-γ和IL-10分泌减少,胸腺、骨髓、脾脏和淋巴结等器官中CD4+T细胞比例均升高,CD8+T细胞比例均下降,表明微囊藻毒素可通过干扰T细胞分化影响机体免疫功能.
综上可知,微塑料和微囊藻毒素等新型环境污染物会扰乱机体免疫系统和激活T细胞.然而,微塑料和微囊藻毒素与Th细胞之间更深入的联系尚未见报道,Th细胞在新型环境污染物诱导的肾脏损伤中作用和机制具有一定的研究前景和现实意义.
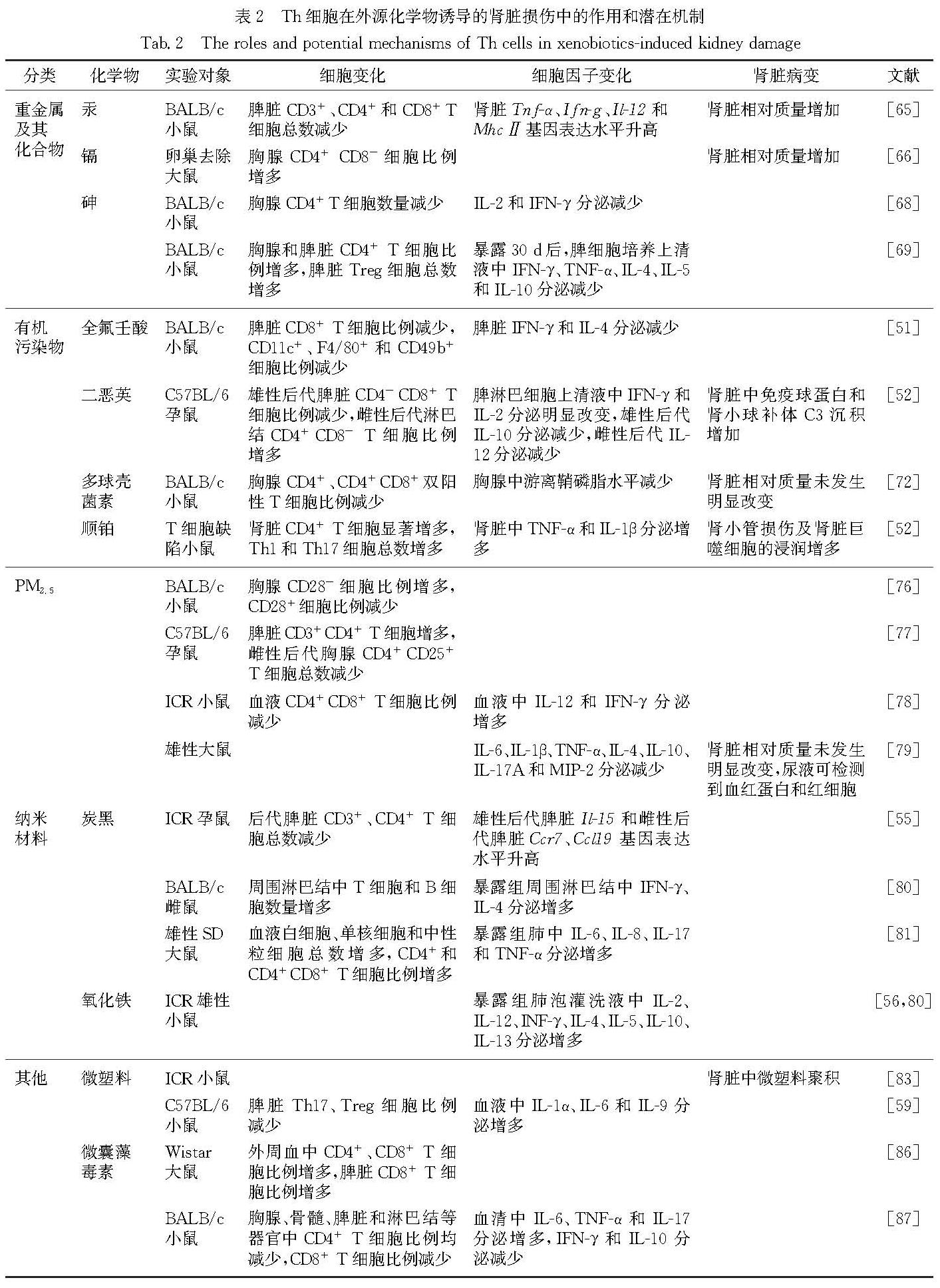
Th细胞可能参与调控不同外源化学物诱导肾脏损伤的作用和潜在机制总结于表2.
3 总结和展望
目前,对外源化学物肾脏免疫毒性的研究主要集中于先天性免疫介导的毒性损伤,而近期发现对于适应性免疫,Th细胞在多种肾脏损伤中发挥重要作用.Th1、Th2、Th17及Treg等多种Th细胞均可参与外源化学物诱导的肾脏免疫毒性损伤.其中,Th1细胞主要介导细胞免疫应答,介导细菌和病毒感染以及其他有毒物质如博来霉素和四氯化碳等胞内病原体的保护性免疫[88]; Th2细胞主要参与体液免疫应答,抵抗变应原、蠕虫和某些真菌等胞外病原体,也可促进过敏和哮喘的发生[89-90].Th1/Th2细胞在功能和细胞转化方面存在此消彼长的相互抑制关系[23-24].此外,肾脏中Th17细胞主要表现为促炎作用,Treg细胞表现为抑炎作用,两者也是相互抑制关系[43-44].外源化学物主要是通过增加Th1或Th17细胞数量,降低Th2或Treg细胞数量,或改变Th1/Th2、Th17/Treg细胞比例而诱导肾脏免疫毒性损伤,因此可认为Th1和Th17细胞主要发挥促进肾脏免疫毒性损伤的作用.利用免疫调节剂干预Th1和Th17细胞数量,或中和性抗体阻断IFN-γ和IL-17,可以恢复Th1/Th2、Th17/Treg细胞比例平衡,改善肾脏免疫微环境,降低外源物毒性损伤[59,64].然而,不同类型Th细胞调控的肾脏免疫毒性作用和机制仍不清楚[91-92],且不断有新分型Th细胞(如Th9和Th22等)被发现,它们在外源化学物肾脏损伤中的作用尚不清楚,是将来一个新的研究方向.此外,不同Th细胞亚群之间,以及它们与先天性免疫细胞之间如何相互调控介导肾脏损伤,也是亟待阐明的科学问题.深入研究外源化学物导致的肾脏损伤中Th细胞的作用和机制,进而调节干预Th细胞,是将来治疗外源化学物诱导肾脏损伤的一种潜在策略.
- [1] TURNER J E,BECKER M,MITTRÜCKER H W,et al.Tissue-resident lymphocytes in the kidney[J].Journal of the American Society of Nephrology,2018,29(2):389-399.
- [2] SUÁREZ-FUEYO A,BRADLEY S J,KLATZMANN D,et al.T cells and autoimmune kidney disease[J].Nature Reviews Nephrology,2017,13(6):329-343.
- [3] ALI A,SUHAIL M,MATHEW S,et al.Nanomaterial induced immune responses and cytotoxicity[J].Journal of Nanoscience and Nanotechnology,2016,16(1):40-57.
- [4] DOLFF S,WITZKE O,WILDE B.Th17 cells in renal inflammation and autoimmunity[J].Autoimmunity Reviews,2019,18(2):129-136.
- [5] STEWART B J,FERDINAND J R,YOUNG M D,et al.Spatiotemporal immune zonation of the human kidney[J].Science,2019,365(6460):1461-1466.
- [6] FANG P,LI X,DAI J,et al.Immune cell subset differentiation and tissue inflammation[J].Journal of Hematology & Oncology,2018,11(1):97.
- [7] MA C S,PHAN T G.Here,there and everywhere:T follicular helper cells on the move[J].Immunology,2017,152(3):382-387.
- [8] UMETSU D T,DEKRUYFF R H.Th1 and Th2 CD4+ cells in human allergic diseases[J].Journal of Allergy and Clinical Immunology,1997,100(1):1-6.
- [9] JÄGER A,DARDALHON V,SOBEL R A,et al.Th1,Th17,and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes[J].The Journal of Immunology,2009,183(11):7169-7177.
- [10] HOPFER H,HOLZER J,HÜNEMÖRDER S,et al.Characterization of the renal CD4+ T-cell response in experimental autoimmune glomerulonephritis[J].Kidney International,2012,82(1):60-71.
- [11] OKAMOTO A,FUJIO K,TSUNO N H,et al.Kidney-infiltrating CD4+ T-cell clones promote nephritis in lupus-prone mice[J].Kidney International,2012,82(9):969-979.
- [12] HAAS C,RYFFEL B,LE HIR M.IFN-γ is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice[J].Journal of Immunology,1997,158(11):5484-5491.
- [13] ZHAO S L,MO Z H,HE H H,et al.Imbalance of T-helper 1/T-helper 2 cytokines and impaired glucose tolerance among patient with acute coronary syndrome[J].Journal of Cancer Research and Therapeutics,2018,14(Sup.1):S480-S485.
- [14] ZHAO Y,JIANG Z S,ZHAO T B,et al.Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells(CB-SCs)in stem cell educator therapy:phase Ⅰ/Ⅱ clinical trial[J].BMC Medicine,2013,11(1):60-69.
- [15] WEN Y,RUDEMILLER N P,ZHANG J D,et al.Stimulating type 1 angiotensin receptors on T lymphocytes attenuates renal fibrosis[J].The American Journal of Pathology,2019,189(5):981-988.
- [16] YOKOTA N,BURNE-TANEY M,RACUSEN L,et al.Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury[J].American Journal of Physiology Renal Physiology,2003,285(2):F319-F325.
- [17] STARK J M,TIBBITT C A,COQUET J M.The metabolic requirements of Th2 cell differentiation[J].Frontiers in Immunology,2019,10:2318.
- [18] KAPLAN M H,SCHINDLER U,SMILEY S T,et al.Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells[J].Immunity,1996,4(3):313-319.
- [19] SELVARAJA M,ABDULLAH M,ARIP M,et al.Elevated interleukin-25 and its association to Th2 cytokines in systemic lupus erythematosus with lupus nephritis[J].PLoS One,2019,14(11):e0224707.
- [20] STANGOU M,BANTIS C,SKOULAROPOULOU M,et al.Th1,Th2 and Treg/T17 cytokines in two types of proliferative glomerulonephritis[J].Indian Journal of Nephrology,2016,26(3):159-166.
- [21] DENG F,ZHANG J W,LI Y,et al.Hirudin ameliorates immunoglobulin a nephropathy by inhibition of fibrosis and inflammatory response[J].Renal Failure,2019,41(1):104-112.
- [22] LIU L,KOU P,ZENG Q,et al.CD4+ T lymphocytes,especially Th2 cells,contribute to the progress of renal fibrosis[J].American Journal of Nephrology,2012,36(4):386-396.
- [23] RAHIMI K,AHMADI A,HASSANZADEH K,et al.Targeting the balance of T helper cell responses by curcu min in inflammatory and autoimmune states[J].Autoimmunity Reviews,2019,18(7):738-748.
- [24] UHM W S,NA K,SONG G W,et al.Cytokine balance in kidney tissue from lupus nephritis patients[J].Rheumatology,2003,42(8):935-938.
- [25] KITCHING A R,HOLDSWORTH S R.The emergence of Th17 cells as effectors of renal injury[J].Journal of the American Society of Nephrology,2011,22(2):235-238.
- [26] MOK M Y,WU H J,LO Y,et al.The relation of interleukin 17(IL-17)and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus[J].The Journal of Rheumatology,2010,37(10):2046-2052.
- [27] JAKIELA B,KOSAKA J,PLUTECKA H,et al.Facilitated expansion of Th17 cells in lupus nephritis patients[J].Clinical and Experimental Immunology,2018,194(3):283-294.
- [28] OREJUDO M,RODRIGUES-DIEZ R R,RODRIGUES-DIEZ R,et al.Interleukin 17a participates in renal inflammation associated to experimental and human hypertension[J].Frontiers in Pharmacology,2019,10:1015.
- [29] GAN P Y,STEINMETZ O M,TAN D S,et al.Th17 cells promote autoimmune anti-myeloperoxidase glome-rulonephritis[J].Journal of the American Society of Nephrology,2010,21(6):925-931.
- [30] MEHROTRA P,PATEL J B,IVANCIC C M,et al.Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT-1R antagonism[J].Kidney International,2015,88(4):776-784.
- [31] CHUNG B H,KIM B M,DOH K C,et al.Protective effect of 1α,25-dihydroxyvitamin D3 on effector CD4+ T cell induced injury in human renal proximal tubular epithelial cells[J].PLoS One,2017,12(2):e0172536.
- [32] DUDAS P L,SAGUE S L,ELLOSO M M,et al.Proinflammatory/profibrotic effects of interleukin-17A on human proximal tubule epithelium[J].Nephron Experimental Nephrology,2011,117(4):e114-e123.
- [33] MARTIN-MORENO P L,TRIPATHI S,CHANDRAKER A.Regulatory T cells and kidney transplantation[J].Clinical Journal of the American Society of Nephrology,2018,13(11):1760-1764.
- [34] ZHU X J,LI S Q,ZHANG Q N,et al.Correlation of increased Th17/Treg cell ratio with endoplasmic reticulum stress in chronic kidney disease[J].Medicine,2018,97(20):e10748.
- [35] WANG G,LAI F M M,TAM L S,et al.Urinary FOXP3 mRNA in patients with lupus nephritis:relation with disease activity and treatment response[J].Rheumatology,2009,48(7):755-760.
- [36] XING Q,WANG B,SU H H,et al.Elevated Th17 cells are accompanied by Foxp3+ Treg cells decrease in patients with lupus nephritis[J].Rheumatology Interna-tional,2012,32(4):949-958.
- [37] GANDOLFO M T,JANG H R,BAGNASCO S M,et al.Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury[J].Kidney International,2009,76(7):717-729.
- [38] KINSEY G R,SHARMA R,HUANG L,et al.Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury[J].Journal of the American Society of Nephrology,2009,20(8):1744-1753.
- [39] YAO L,CHEN H P,MA Q.Piperlongumine alleviates lupus nephritis in MRL-FAS(LPR)mice by regulating the frequency of Th17 and regulatory T cells[J].Immunology Letters,2014,161(1):76-80.
- [40] NADKARNI S,MAURI C,EHRENSTEIN M R.Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β[J].The Journal of Experimental Medicine,2007,204(1):33-39.
- [41] TEJERA-ALHAMBRA M,ALONSO B,TEIJEIRO R,et al.Perforin expression by CD4+ regulatory T cells increases at multiple sclerosis relapse:sex differences[J].International Journal of Molecular Sciences,2012,13(6):6698-6710.
- [42] ZHANG C,WANG S,LI J,et al.The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury[J].Cell Death & Disease,2017,8(3):e2695.
- [43] WANG Y M,GHALI J,ZHANG G Y,et al.Development and function of Foxp3+ regulatory T cells[J].Nephrology,2016,21(2):81-85.
- [44] LUO T,JI W J,YUAN F,et al.Th17/Treg imbalance induced by dietary salt variation indicates inflammation of target organs in humans[J].Scientific Reports,2016,6(1):26767.
- [45] LI Y,SHI Y Y,HUANG Z C,et al.CNI induced Th17/Treg imbalance and susceptibility to renal dysfunction in renal transplantation[J].International Immunopharma-cology,2011,11(12):2033-2038.
- [46] 王心如.毒理学基础[M].李焕德,译.6版.北京:人民卫生出版社,2014:14.
- [47] SINGH N,KUMAR A,GUPTA V K,et al.Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations[J].Chemical Research in Toxicology,2018,31(10):1009-1021.
- [48] RANA M N,TANGPONG J,RAHMAN M M.Toxico-dynamics of lead,cadmium,mercury and arsenic-induced kidney toxicity and treatment strategy:a mini review[J].Toxicology Reports,2018,5:704-713.
- [49] GUO W J,PAN B H,SAKKIAH S,et al.Persistent organic pollutants in food:contamination sources,health effects and detection methods[J].International Journal of Environmental Research and Public Health,2019,16(22):E4361.
- [50] WANG H,ZHANG J X,YE L-P,et al.Plasma Kallikrein-Kinin system mediates immune-mediated renal injury in trichloroethylene-sensitized mice[J].Journal of Immuno-toxicology,2016,13(4):567-579.
- [51] FANG X M,ZHANG L J,FENG Y X,et al.Immunotoxic effects of perfluorononanoic acid on BALB/c mice[J].Toxicological Sciences,2008,105(2):312-321.
- [52] MUSTAFA A,HOLLADAY S D,GOFF M,et al.An enhanced postnatal autoimmune profile in 24 week-old C57BL/6 mice developmentally exposed to TCDD[J].Toxicology and Applied Pharmacology,2008,232(1):51-59.
- [53] CARLISLE A J,SHARP N C.Exercise and outdoor ambient air pollution[J].British Journal of Sports Medicine,2001,35(4):214-222.
- [54] HEIDAI-MOGHADAM A,KHORSANDI L,JOZI Z.Curcu min attenuates nephrotoxicity induced by zinc oxide nanoparticles in rats[J].Environmental Science and Pollution Research,2019,26(1):179-187.
- [55] SHIMIZU R,UMEZAWA M,OKAMOTO S,et al.Effect of maternal exposure to carbon black nanoparticle during early gestation on the splenic phenotype of neonatal mouse[J].The Journal of Toxicological Sciences,2014,39(4):571-578.
- [56] PARK E J,KIM H,KIM Y,et al.Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice[J].Toxicology,2010,275(1/2/3):65-71.
- [57] ANDRADY A L.Microplastics in the marine environment[J].Marine Pollution Bulletin,2011,62(8):1596-1605.
- [58] BROWNE M A,CRUMP P,NIVEN S J,et al.Accumulation of microplastic on shorelines worldwide:sources and sinks[J].Environmental Science & Technology,2011,45(21):9175-9179.
- [59] LIMONTA G,MANCIA A,BENKHALQUI A,et al.Microplastics induce transcriptional changes,immune response and behavioral alterations in adult zebrafish[J].Scientific Reports,2019,9(1):15775.
- [60] MCLELLAN N L,MANDERVILLE R A.Toxic mechanisms of microcystins in mammals[J].Toxicology Research,2017,6(4):391-405.
- [61] 孟紫强.现代环境毒理学[M].北京:中国环境出版社,2015:413-740.
- [62] TE J A,ABDULHAMEED M D M,WALLQVIST A.Systems toxicology of chemically induced liver and kidney injuries:histopathology-associated gene co-expression modules[J].Journal of Applied Toxicology,2016,36(9):1137-1149.
- [63] NASCIMENTO S N,GÖETHEL G,BAIERLE M,et al.Environmental exposure and effects on health of children from a tobacco-producing region[J].Environ-mental Science and Pollution Research,2017,24(3):2851-2865.
- [64] LANGWORTH S,ELINDER C G,SUNDQUIST K G,et al.Renal and immunological effects of occupational exposure to inorganic mercury[J].British Journal of Industrial Medicine,1992,49(6):394-401.
- [65] KIM S H,JOHNSON V J,SHARMA R P.Oral exposure to inorganic mercury alters T lymphocyte phenotypes and cytokine expression in BALB/c mice[J].Archives of Toxicology,2003,77(11):613-620.
- [66] PILLET S,D'ELIA M,BERNIER J,et al.Immunomodulatory effects of estradiol and cadmium in adult female rats[J].Toxicological Sciences,2006,92(2):423-432.
- [67] RAQIB R,AHMED S,SULTANA R,et al.Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh[J].Toxicology Letters,2009,185(3):197-202.
- [68] MORZADEC C,BOUEZZEDINE F,MACOCH M,et al.Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes[J].Toxicology,2012,300(1/2):46-56.
- [69] GERA R,SINGH V,MITRA S,et al.Arsenic exposure impels CD4 commitment in thymus and suppress T cell cytokine secretion by increasing regulatory T cells[J].Scientific Reports,2017,7(1):7140.
- [70] COUTINHO J V S,FREITAS-LIMA L C,FREITAS F F C T,et al.Tributyltin chloride induces renal dysfunction by inflammation and oxidative stress in female rats[J].Toxicology Letters,2016,260:52-69.
- [71] JOHNSON V J,HE Q R,OSUCHOWSKI M F,et al.Disruption of sphingolipid homeostasis by myriocin,a mycotoxin,reduces thymic and splenic T-lymphocyte populations[J].Toxicology,2004,201(1/2/3):67-75.
- [72] VOLAREVIC V,MARKOVIC B S,JANKOVIC M G,et al.Galectin 3 protects from cisplatin-induced acute kidney injury by promoting TLR-2-dependent activation of IDO1/Kynurenine pathway in renal DCs[J].Theranostics,2019,9(20):5976-6001.
- [73] LEE H,NHO D,CHUNG H S,et al.CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice[J].Kidney International,2010,78(11):1100-1109.
- [74] RAN J J,YANG A M,SUN S Z,et al.Long-term exposure to ambient fine particulate matter and mortality from renal failure:a retrospective cohort study in Hong Kong,China[J].American Journal of Epidemiology,2020,189(6):602-612.
- [75] LIU C,XU J,CHEN Y,et al.Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature[J].Environ-mental Health:A Global Access Science Source,2015,14:65.
- [76] DRELA N,ZES'KO I,JAKUBOWSKA M,et al.CD28 in thymocyte development and peripheral T cell activation in mice exposed to suspended particulate matter[J].Toxicology and Applied Pharmacology,2006,215(2):179-188.
- [77] CHEN L,BENNETT E,WHEELER A J,et al.Maternal exposure to particulate matter alters early post-natal lung function and immune cell development[J].Environmental Research,2018,164:625-635.
- [78] PARK E J,ROH J,KIM Y,et al.PM2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice[J].Environmental Research,2011,111(3):348-355.
- [79] AZTATZI-AGUILAR O G,URIBE-RAM REZ M,NARV EZ-MORALES J,et al.Early kidney damage induced by subchronic exposure to PM2.5 in rats[J].Particle and Fibre Toxicology,2016,13(1):68-75.
- [80] PARK E J,OH S Y,LEE S J,et al.Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells[J].Environmental Research,2015,143(PtA):138-147.
- [81] CHU C,ZHOU L X,XIE H R,et al.Pulmonary toxicities from a 90-day chronic inhalation study with carbon black nanoparticles in rats related to the systemical immune effects[J].International Journal of Nanomedicine,2019,14:2995-3013.
- [82] VAN ZIJVERDEN M,VAN DER PIJL A,BOL M,et al.Diesel exhaust,carbon black,and silica particles display distinct Th1/Th2 modulating activity[J].Toxicology and Applied Pharmacology,2000,168(2):131-139.
- [83] DENG Y,ZHANG Y,LEMOS B,et al.Tissue accu-mulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure[J].Scientific Reports,2017,7:46687.
- [84] LI B Q,DING Y F,CHENG X,et al.Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice[J].Chemosphere,2020,244:125492.
- [85] YI X P,XU S S,HUANG F Y,et al.Effects of chronic exposure to microcystin-LR on kidney in mice[J].International Journal of Environmental Research and Public Health,2019,16(24):5030.
- [86] PALIKOVA M,ONDRACKOVA P,MARES J,et al.In vivo effects of microcystins and complex cyanobacterial biomass on rats(Rattus norvegicus var.alba):changes in immunological and haematological parameters[J].Toxicon,2013,73:1-8.
- [87] DAR H Y,LONE Y,KOIRI R K,et al.Microcystin-leucine arginine(MC-LR)induces bone loss and impairs bone micro-architecture by modulating host immunity in mice:implications for bone health[J].Environmental Pollution,2018,238:792-802.
- [88] WILSON M S,MADALA S K,RAMALINGAM T R,et al.Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent[J].The Journal of Experimental Medicine,2010,207(3):535-552.
- [89] GIESECK R L,WILSON M S,WYNN T A.Type 2 immunity in tissue repair and fibrosis[J].Nature Reviews Immunology,2018,18(1):62-76.
- [90] WYNN T A.Fibrotic disease and the Th1/Th2 paradigm[J].Nature Reviews Immunology,2004,4(8):583-594.
- [91] BENNINGHOFF A D,BATES M A,CHAUHAN P S,et al.Docosahexaenoic acid consumption impedes early interferon-and chemokine-related gene expression while suppressing silica-triggered flaring of murine lupus[J].Frontiers in Immunology,2019,10:2851.
- [92] WU H J,LIAO W,LI Q W,et al.Pathogenic role of tissue-resident memory T cells in autoimmune diseases[J].Autoimmunity Reviews,2018,17(9):906-911.